Tracking Designer Drugs
Combining solid phase extraction with high-resolution accurate mass LC-MS increases sample throughput and improves detection and identification of novel synthetic cannabinoids.
The Problem
The rapid increase of novel and variant synthetic cannabinoids designed to circumvent regulation has resulted in many unknown analytes and metabolites that must be detected in forensic and toxicological urine samples. How can we more capably detect trace amounts of these drugs in complex matrices, such as urine and blood?
Background
In recent years, many designer drugs have become available for purchase online and in specialist shops. A subset of these compounds – synthetic cannabinoids – are marketed as herbal smoking blends under the generic name ‘Spice’. Others are marketed under the guise of incense or room odorizers but, despite often being marked as “not for human consumption”, are smoked as an alternative to cannabis. Spice products tend to profess a long list of plant or herbal ingredients and when previously analysed were not found to contain tobacco or cannabis. However, more detailed investigation of these herbal mixtures (1, 2) have identified unlisted active ingredients such as JWH-018 and CP47,497 (see Figure 1). Such drugs are classified as cannabinoids because they invoke similar physiological and psychological effects to active ingredients in cannabis, for example tetrahydrocannabinol (THC). Many synthetic cannabinoids were originally developed by John W. Huffman’s group in Clemson University, South Carolina, in the 1980s-90s to explore drug-receptor interactions targeting the CB1 and CB2 cannabinoid receptors (2, 3), but most have never been tested on humans. Whereas the side effects of cannabis are well documented, there is a general lack of information regarding the exact ingredients, purity, potency and long term effects of synthetic cannabinoid mixtures. In fact, the use of synthetic cannabinoids has been shown to cause severe withdrawal, psychotic episodes, convulsions, and life-threatening conditions in some individuals (4, 5).
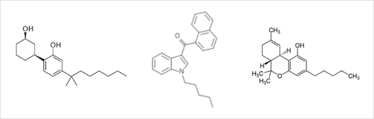
Fig 1: Structures of the synthetic cannabinoids CP47, 497 (left) and JWH-018 (centre), and the structure of tetrahydrocannabinol (THC), the major active compound in cannabis (right).
As a consequence of these health risks, the sale of cannabinoids has become increasingly controlled by authorities worldwide (6). However, almost as quickly as legal restrictions are placed upon drugs, many isomers, derivatives, and novel variants are developed to replace them, with just small changes in structure required to put molecules outside of the scope of standard detection. With a constant influx of novel molecules, there is a clear need for the ability to detect trace levels of both known and novel compounds for forensic and toxicological purposes. But how can we go about doing this?
Synthetic cannabinoids can be resolved and detected using routine gas chromatography(GC)-mass spectrometry (MS) and liquid chromatography (LC)-MS, but identification and quantitation is limited by the availability of pure reference materials. Tandem LC-MS coupled with analyte specific transitions can assist with detection where metabolites are known and reference materials are available to optimize transitions, but where reference materials are not available and metabolism is unknown, tandem LC-MS approaches are of less use.
The Solution
To combat the rapid increase in variants and novel cannabinoids that fall outside the current legislation, we have employed high resolution accurate mass (HRAM) LC-MS (see the "The System", page 2). The use of this technology greatly improves the speed, selectivity and capacity of drug detection in urine samples from suspected users. Whereas numerous compounds of interest with differing chemical formulae may have the same nominal mass, their accurate masses are different and resolvable by HRAM LC-MS. High selectivity in full scan mode, coupled with low retention time, potentially allows hundreds of compounds to be accurately identified. MS/MS can then be used to elucidate structures of isomers, further increasing selectivity and reducing the need for reference materials to set up a screen.
Although selectivity is greatly improved by HRAM LC-MS, injection of unextracted urine results in substantial ion suppression and matrix interference. In this case, trace levels of cannabinoids may remain undetected so we developed a solid phase extraction (SPE) procedure to purify and concentrate samples prior to analysis. The specific interaction between target analytes and the chosen sorbent bed permits various ‘washes’ of chosen polarity, acidity, and aqueous/organic constituents to remove as much matrix as possible before elution of the target compounds. Synthetic cannabinoids generally undergo extensive phase I and II metabolism, resulting in glucuronic acid-conjugated hydroxylated metabolites. Therefore, the SPE procedure was preceded by an enzymatic hydrolysis step using β-glucuronidase to cleave sugars from the drug molecule.
To prepare urine for extraction, five synthetic cannabinoids (JWH-018, JWH-073, JWH-200, JWH-250 and AM-694) were spiked at 50 ng/ml into blank urine. Additionally, an unextracted standard of the five cannabinoids in 10% MeOH in H2O was prepared to determine sample recovery during extraction.
Samples were extracted by SPE and analysed using full scan HRAM LC-MS, with data processing to screen for known compounds. Follow-up on suspect analytes was then conducted using the same sample but with MS/MS.
Cannabinoid recovery was highly consistent between different urine samples, (80–90 percent), with highest recoveries of 96 percent compared with the unextracted standard. Peak responses were strong and we decided that the spiking level in subsequent analyses could be dropped by a factor of 20, giving a new reporting level of 2.5 ng/ml.
The assay was then tested in parallel on urine samples from a hospital patient suspected of consuming herbal smoking mixtures. As administration samples would contain predominantly phase I and II metabolites, it was decided that any of the tested compounds could be used as an internal marker. Therefore, a 2 ml aliquot of the urine was spiked with JWH-018, hydrolyzed, and extracted by SPE.
The output from the ToxID software library suggested detection of a hydroxylated metabolite of JWH-122 and one of JWH-018, alongside the JWH-018 internal marker (see Figure 2). MS/MS data confirmed the structures were most likely those suggested by the library (see Figure 3a and 3b). However, interrogation of the full-scan MS data uncovered the presence of further suspect compounds. MS/MS data confirmed these as one metabolite of AM-2201 (see Figure 3c) and two metabolites of MAM-2201 (see Figure 3d and 3e).
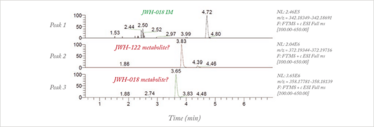
Fig 2: ToxID output from a hospital patient urine sample, suggesting two synthetic cannabinoids are present alongside the internal marker.
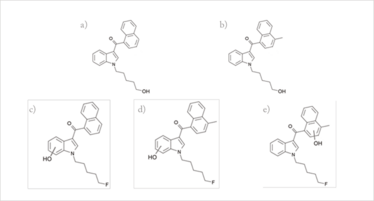
Fig 3: Structures of five metabolites detected in a hospital patient urine sample. (a) A structure identified by the ToxID library as hydroxy JWH-018, but is likely to be a metabolite of AM-2201, with hydroxylation occurring by substitution of the fluorine atom on the alkyl side chain. (b) A structure identified by the ToxID library as hydroxy JWH-122, but, as with 3(a), it is likely to be a metabolite of MAM-2201. (c) Hydroxy metabolite of AM-2201 with hydroxylation on the indole moiety. (d) Hydroxy metabolite of MAM-2201 with hydroxylation on the indole moiety. (e) Hydroxy metabolite of MAM-2201 with hydroxylation on the naphthoyl moiety.
The five metabolites initially suggested that four compounds were present in the urine sample. However, hydroxylation of cannabinoid alkyl side chains can also occur (7). So, while it is possible that the sample contained all four compounds, it is far more likely that they are instead all metabolites of AM-2201 and MAM-2201.
The System
Hardware: Thermo Accela LC system with Phenomenex Luna C18 (2) column, interfaced to Thermo LTQ Orbitrap, providing 6-minute run time.
Software: Full scan HRAM LC-MS data processed and analyzed by ToxID (Thermo Fisher). ToxID’s database is regularly updated and includes information on protonated monoisotopic masses of known cannabinoids. Narrow range mass filters (typically ±1 ppm) can be employed to selectively filter data for reporting. Analysis is further aided by entering retention times for analytes for which reference material has been obtained or for which prior detection has occurred. Such processing allows retrospective screening of prior-run samples once new compounds are detected and added to database.
Previous work on the metabolism of JWH-018 has demonstrated multiple hydroxy metabolites and similar metabolism has also been observed for JWH-122. Our sample had only one metabolite for both JWH-018 and JWH-122, which is more consistent with them being derived from AM-2201 and MAM-2201 respectively, where hydroxylation is occurring via substitution of the fluorine atom. For AM-2201, substitution was also detected on the indole moiety. MAM-2201 had hydroxylation on both the naphthoyl and the indole moieties.
Identification and reporting of unknown metabolites in a case like this would be virtually impossible without HRAM LC-MS. The method employed is selective and sensitive, and provides affordable, rapid turnaround times for synthetic cannabinoid screens of both blood and urine samples.
Beyond the solution
Following the identification of five new metabolites of AM-2201 and MAM-2201, the ToxID library database was updated to include the compounds for future testing – the database currently screens for over 180 cannabinoids and their metabolites. It also makes retroactive screening of previously analyzed samples possible. Most importantly, it offers broad coverage and the ability to respond rapidly to new compounds because the database can be regularly updated with new, internationally reported compounds.
Dave Strong is a scientist and Simon Hudson is a technical manager at HFL Sport Science, Cambridgeshire, UK.
- V. Auwärter et al., “‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs?” J. Mass Spectrom., 44, 832 (2009).
- N. Uchiyama et al., “Identification of a cannabinoid analog as a new type of designer drug in a herbal product”, Chem. Pharm. Bull., 57, 439 (2009).
- P. I. Dargan et al., “The impact of changes in UK classification of the synthetic cannabinoid receptor agonists in ‘Spice’”. Int. J. Drug Policy, 22 (4), 274 (2011).
- U. S. Zimmermann et al., “Withdrawal phenomena and dependence syndrome after the consumption of ‘Spice Gold’. Dtsch Arztebl Int. 2009, 106, 464.
- J. Lapoint et al., “Severe toxicity following synthetic cannabinoid ingestion”, Clin. Toxicol. (Phila.), 49(8), 760 (2011).
- Advisory Council on the Misuse of Drugs, Consideration of the major cannabinoid agonists. Home Office, London, 2009. Available at: bit.ly/15KHhxm. Accessed 15 July 2013.
- T. Sobolevsky, I. Prasolov, and G. Rodchenkov, “Detection of JWH-018 metabolites in smoking mixture post-administration urine”, Forensic Sci. Int., 200, 141 (2010).
Dave Strong graduated with a PhD in analytical chemistry from the University of Bristol, UK, in 2012 and currently performs toxicological testing on human samples at HFL Sports Science. “My work entails extensive research and method development in both biological and man-made matrices, which includes substantial wet chemistry, such as solid phase extraction”. Dave has developed numerous methods from scratch, such as the extraction of caffeine from saliva, the extraction of octopamine from human urine…