Perfect Geometry
Does UniSpray’s aerodynamic design give it the edge over conventional electrospray ionization?
The problem
Ionization is a critical process in mass spectrometry. All mass spectrometers – no matter how powerful or sophisticated – are highly dependent on the efficient generation of gas phase sample ions in the ion source. Electrospray ionization (ESI) sources have been widely adopted since their commercialization in the late 1980s, and have become the first choice for most day-to-day analyses. However, all ionization mechanisms have limitations; ESI ionization efficiency is compromised at high flow rates and when using highly aqueous mobile phases. To analyze the broadest range of different molecules, analysts must select the most appropriate source and potentially switch sources during analyses.
Background
Early commercial ESI sources allowed mass spectrometry (MS) to be applied to biological compounds, such as peptides and proteins, which would typically be infused into the source at low flow rates of 1–5 μL/min. Under these low flow conditions, atomization was achieved via a classical Taylor cone (formed at the end of the liquid capillary), following the application of a few kilovolts between the capillary and the ion inlet cone. More recently, infusion-based analysis for biological applications has been superseded by nanospray ESI capillaries that operate at extremely low flow rates (10–1000 nL/min) and offer high ionization efficiency for small sample volumes.
From a commercial viewpoint, the greatest leap in ESI sources came from the marriage of mass spectrometry and liquid chromatography (LC-MS), which gave analytical chemists access to the enhanced specificity offered by both techniques. To adapt ESI sources to the high flow rates (0.1–1.0 mL/min) typically used in LC (or its modern equivalent UPLC), the atomization process was boosted by the addition of a concentric flow of high-velocity nitrogen gas at the ESI tip. However, when conducting infusion experiments with a fixed analyte concentration from flow rates of 10 nL/min to 1 mL/min, it is common to see the analyte signal increase by a factor of 20 at the highest flow rates, while analyte consumption jumps by a factor of 100,000. The cause? The poor ionization efficiency of high flow rate ESI, which is critically dependent on droplet factors, such as size distribution, charge per unit volume and evaporation rates, as well as additional factors, including inlet sampling efficiency. Ionization efficiency can become particularly challenging when using highly aqueous mobile phases.
The solution
There is a body of historical evidence to suggest that surfaces play an important role in both the sensitivity and stability of atmospheric pressure ionization sources such as ESI. Although the reasons were not understood, we knew that high velocity sprays that impinge on curved surfaces, in particular, could improve source performance. The ion source described in this article originated from experiments that were designed to optimize these effects and the associated requirement for efficient coupling with the ion inlet of the mass spectrometer.
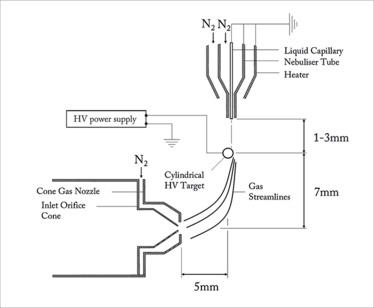
Figure 1. A schematic of the UniSpray API source
Work started in early 2011 at Waters’ MS Research Centre. In these early experiments, an insulated, tapered high voltage rod target was manipulated three-dimensionally via a microadjuster in the space between the sprayer and the ion inlet cone. The use of a tapered target allowed us to rapidly and accurately determine the effect of target diameter on ion signal. It very rapidly became apparent that significant enhancements in ion signal could be achieved compared to conventional high flow rate ESI by carefully optimizing the geometrical parameters of the experimental arrangement. As a result of this, we found that we were able to increase ionization efficiency by combining a high-velocity spray with a high-voltage, cylindrical target that is positioned in an off-axis, cross-flow arrangement, immediately upstream of the ion sampling cone – we called this novel ionization technique UniSpray™.
UniSpray shares some features with high flow rate ESI sources, in that the liquid flow is nebulized by a high-velocity, concentric nitrogen gas flow. However, unlike ESI, the high voltage is applied to a cylindrical target electrode that is positioned close to the grounded nebulizer, such that the near-supersonic jet impinges on the target surface. A schematic of the UniSpray source is shown in Figure 1. The source is formed by directing a high-velocity, nebulized jet from a grounded sprayer onto a cylindrical metal target that is held at a high voltage and is located between the sprayer and the ion inlet orifice of the mass spectrometer. Nitrogen gas is delivered to the nebulizer tube at a gauge pressure of 7 bar. Under these conditions, the nitrogen jet will be near-supersonic at a distance of a few millimeters from the nebulizer tip.
To optimize source sensitivity, it is critical that the collimated spray impacts on exactly the right point on the cylindrical target. As shown in Figure 1, the maximum signal intensity is typically obtained when the spray is asymmetrically positioned to hit the upper right quadrant of the target. Under these conditions, the gas flow becomes attached to a portion of the curved surface and results in asymmetric gas streamlines in the wake, which are directed towards the ion inlet orifice. This flow phenomenon is known as the Coanda effect. Under the influence of the Coanda flow field, ions and charged droplets are directed towards the ion inlet, which is surrounded by a cone gas nozzle that supports a drying gas flow of nitrogen at 150 L/hr.
UniSpray sources generally enhance ionization efficiency when compared to high flow rate ESI, which can in turn enhance analytical sensitivity. Some general characteristics of the UniSpray source have been studied by Lubin et al., who compared the signal intensity obtained by ESI and UniSpray for small pharmaceutical compounds (16 analytes in positive ion mode and 7 in negative ion mode) with acidic, basic and neutral mobile-phase types (1). Figure 2 shows the signal gains (UniSpray ion intensity divided by ESI ion intensity) obtained at various mobile-phase compositions and for three different flow rates. Here, each point is an average of the pooled data for each compound and each mobile-phase type. From these plots it can be concluded that the signal intensity observed with the UniSpray source is higher on average than the ESI signal for all mobile-phase compositions, with the greatest gains seen under highly aqueous conditions. UniSpray gains in excess of ×20 were observed under certain conditions, but gains were highly compound-specific, with a few analytes giving greater responses with an ESI source.
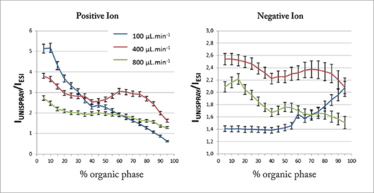
Figure 2 – A comparison of the average signal intensity ratios for UniSpray and ESI versus mobile phase composition for 16 positive ion analytes and 7 negative ion analytes (from reference [1]).
The unique geometry of the UniSpray ion source generates several different mechanisms to produce smaller droplets and enhance desolvation. You can read more about these underlying mechanisms at www.waters.com/unispraymechanisms.
Beyond the solution
UniSpray has the potential to increase the number and type of compounds that can be detected by mass spectrometry in a single run and simultaneously enhance sensitivity. Therefore, UniSpray could allow the consolidation of multiple methods into a single analysis, optimizing laboratory efficiency, and allowing users to see a more complete picture of their samples. Work is ongoing to characterize the behavior of the UniSpray source over a wide range of analyte types, mobile phases and additives for both UPLC and UPC2 applications. I also believe that, as the technology becomes widespread, UniSpray will expand into diverse areas, such as biomolecules, oilfield samples and the ambient ionization of challenging analytes.
Steve Bajic is a Senior Research Scientist at Waters Corporation, Wilmslow, UK.
- Lubin et al., “Atmospheric pressure ionization using a high voltage target compared to electrospray ionization”, J Am Soc Mass Spectrom, 28, 286–293 (2017).
Steve Bajic is a Senior Research Scientist at Waters Corporation, Wilmslow, UK.