A Briefcase Full of NMR
One half of The Blues Brothers – Bernhard Blümich, a leading expert in compact nuclear magnetic resonance (NMR) spectroscopy – shares the story behind the innovative technology that is enabling scientists to perform spectroscopic analyses in minutes instead of hours or even days.

In the late 1940s and early 1950s, NMR spectroscopy opened up a new world of knowledge (1). Today’s impressive high-field instruments use powerful, helium-cooled superconducting magnets to provide unprecedented resolution – in other words, they are expensive and large, which restricts their use to specialized facilities with plenty of floor space. It took a brainstorming session and a simple question about simplicity to spur development in a different direction – downsizing.
Call them portable, benchtop or compact, these smaller devices are cheaper, they are robust and they can be moved from bench to bench. And, though they are clearly not a replacement for the larger scale systems required for investigation of complex molecules, portability has made NMR spectroscopy more accessible to a wider range of scientific investigators.
Indeed, the greater availability of benchtop, transportable instruments is opening up new opportunities for biochemistry and organic chemistry, quality control and chemical reaction monitoring, where smaller molecules are the target. And recent advances in miniaturizing magnet and radio frequency (RF) spectrometer electronics point the way to truly portable NMR spectroscopy (2).
“Compared with high-field NMR, the benefit of benchtop NMR is the speed that you get results. It takes just a few minutes, including sample preparation,” says Bernhard Blümich, a professor and chair of macromolecular chemistry at RWTH Aachen University, Germany, and one of the leading experts in the field.
“Traditionally, with high-field NMR spectroscopy the user prepares a sample, puts it into a sample tube, sends it to the NMR lab (usually down in the basement of the building) where it goes into a sample changer. The sample eventually gets measured. And, if they’re good results, they’ll appear on the intranet so that the chemist can check whether the reaction went the right way. All of that typically takes half a day, sometimes a full day,” says Blümich, who points out that he has an advisory and commercial interest in Magritek, a company that specializes in cryogen-free, compact NMR and magnetic resonance imaging (MRI) systems.
On a mission from God?
Let’s go back to 1993 and a brainstorming session between “The Blues Brothers” – as Blümich and his friend Peter Blümler were known (Blümler is currently a senior scientist in the Institute of Physics, at Johannes Gutenberg University in Mainz, Germany).
“Peter is extremely creative and out of our brainstorming came the question: ‘Why can’t we simplify NMR?’ The manufacturers at that time were all building instruments using superconducting magnets, aiming for higher and higher field strengths and they were complicated – they still are. We started to consider the simplest way to get an NMR signal. Admittedly, we hadn’t given much thought to applications at that stage. Peter drew a design and that led to the development of a small sensor now known as the NMR-Mouse,” says Blümich. Mouse stands for “mobile universal surface explorer.”
A few years later, after that initial mind-melding session, Blümich moved to the university in Aachen and hired Blümler to work in his lab.
“The Blues Brothers were back together and our first project was to actually build the NMR-MOUSE. We got our first signals from that initial instrument in 1995. The sensor uses what is generically called stray-field NMR and we used it to test various materials. We looked at the signals and investigated their differences – and we also looked at increasing sensitivity so that we could measure faster. In the early days, it could take 20 minutes to get a signal, which was impractical but fine for our investigations. It kept our students enthusiastic and curious, because it was a fun thing to do,” says Blümich.
Funding the compact revolution
The German Research Society funded Blümich’s group investigation into single unilateral imaging – or single-sided MRI – which gave them some financial security, although he now views the technique as obsolete.
“Single-sided MRI as far as I am now concerned is a dead end. It’s interesting scientifically, but from a practical point of view it is useless. We then received funding through a European Community Project on Cultural Heritage award, where the NMR-MOUSE again came into play for investigating works of art, which was published in Scientific American (3),” he says.
Because of the funding, the group was able to improve the technique, plus Blümich reports that he was fortunate in having some very good students who were able to optimize the sensor’s stray field shape. Blümich started up a small company to manufacture the NMR-MOUSE, to meet demand for the device. Much has been done to reduce the size of the sensor, which is now available in sizes of less than 2 cm3 – and they are likely to be even smaller in the future...“We kept looking forward and our next step was to build a permanent magnet suitable for spectroscopy. It had been done before, but the magnets were huge. Varian, for example, had produced a beautiful machine, but it was cubic meter in size, so it was more floortop than benchtop... Our aim was to build a much smaller device, which we thought would be feasible if we could get the magnet right," says Blümich.
“We needed to know whether we could build a small magnet with a homogeneous field. Now, many will say that you can’t have a homogeneous stray field field outside of the magnet, but that’s not completely true or, in practical terms, relevant. What you really need is sufficient homogeneity to achieve good chemical analysis. It doesn’t have to be a completely homogeneous field; it just needs to be good enough.”
Sheer magnetism
Federico Casanova and Juan Perlo, two of Blümich’s post-doctoral researchers, built a small magnet that measured no larger than a C-size torch battery and could be used with a 5 mm sample tube (4). The magnet had a field strength of 27 MHz proton NMR frequency, which could be adjusted using shims, meaning that such a permanent magnet could be used to perform spectroscopy.
“Magnets aren’t expensive and are readily available from many sources in China; however, the big issue is quality control. Every magnet with supposedly the same specifications will vary in geometry and size. Also, the granular structure of the sintered material used to manufacture the magnet will vary, which affects the direction and magnitude of magnetization. And that affects homogeneity,” says Blümich.
Apparently, the best way to build a magnet to overcome these problems is to use the design proposed by Klaus Halbach back in 1980 (5). Blümich adds, "The best homogeneity for a magnet is 10-4 and we need this to be 10-8 for spectroscopy – which means the magnetic field across your sample tube should not vary more than 10-8 of your total magnetic field. As scientists we deal with numbers such as 10-23 and so on. But, 10-8 is like building a 100 km long x 1 mm thick road or having the ability to spot a truck on the moon. So that’s the type of precision we want. Of course, you can do this with big superconducting magnets – but Federico and Juan achieved it with a battery-sized permanent magnet based on Halbach’s design.”
Success breeds success
Casanova and Perlo’s magnetic prowess led to the formation of a new business called Magritek, which Blümich set up with the late Sir Paul Callaghan. He developed the electronics in New Zealand, while the group in Aachen continued to develop the magnet.
Today, desktop spectroscopy is not better than high-field spectroscopy in terms of resolution, chemical shift and dispersion, but it does offer features that the latter lacks, Blümich says. “You can take a benchtop instrument and put it inside a fume cupboard to observe a hazardous reaction as it happens. You can’t do that with a high-field machine down in the basement of a building – the lack of convenience, the fact that you’d hold everyone else up and the expense of replacing the spectrometer if something goes wrong, make it impractical.
“So, the beauty of these small devices is the ability to do reaction monitoring in situ – and that saves a lot of time and effort when performing product, quality or reaction control during synthesis. It takes only 10 minutes from sample preparation to obtaining the results to do – the spectrum takes only one minute to develop. That has to be interesting for both academics and scientists working in industrial labs.”
Blümich says the sensitivity of compact instruments is constantly improving and he says achieving homogeneity is the key to this. “Some say that sensitivity is proportional to field strength, but I believe homogeneity is more important. Think again about the truck on the moon – I may be able to see the whole, but how much of it can I see in detail? With improved homogeneity, comes better discrimination of the molecules or chemical groups of interest.
“And chemists don’t really care about your spectrums or if there are some effects going to different fields; he or she just wants to know how many scans will it take to measure the substance and how long it will take to do the experiment and whether all the necessary information will be available. That comes with homogeneity.”
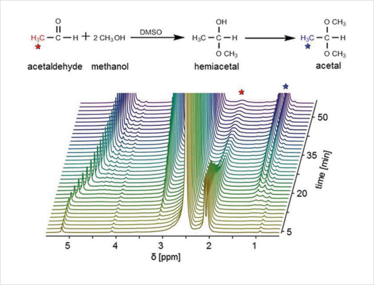
Figure 1. Acetylization spectrum obtained from benchtop NMR spectrometer.
Finding The Holy Grail
Carbon-13 was discovered in 1945 (6) and 13C spectroscopy was still seen as miraculous when Blümich began his PhD in 1977. Today, it’s just a commonplace feature of modern high-field machines. Now, it is also possible to perform it with a benchtop device.
“We’ve been able to measure 13C spectra with the Magritek, which is unusual; we’re also able to produce two-dimensional NMR spectra. Ultimately, what the chemist needs out of a workhorse benchtop machine is the ability to get more than one-dimensional proton spectra. So, they will do a simple analysis, then run a 13C spectrum – and if they want even more they do a 2D or even a DEPT spectrum, which will edit the 13C spectrum into the different chemical groups,” says Blümich. “Eventually, small desktop spectrometers will cover more and more of the ground that high-field machines are used for today. However, the very high-sensitivity of high-field machines will be maintained as they are needed for large molecule work, such as protein structure determination. But, science is full of surprises, so who really knows what we shall see in the future; three years ago no one would have believed that 13C 2D spectroscopy would be possible on a benchtop machine.”
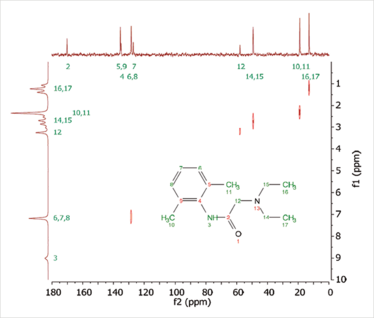
Figure 2. HETCOR spectrum of 1 M lidocaine in CDCl3 obtained from benchtop NMR spectrometer.
Looking to the future
Blümich sees a very clear path for future developments worldwide, with smaller magnets enabling the manufacture of even smaller and more powerful spectrometers. “I’m relatively cautious with my predictions, but I am intrigued by Google[x] Life Sciences. Two NMR specialists – Vik Bajaj and Vasiliki (Vicky) Demas – are leading the team. Vicky in fact did part of her PhD in my group and researched portable magnetic resonance (MR) and ultra-low field MR imaging (MRI) in the laboratories of Alexander Pines, Jeffrey Reimer, and Robert Maxwell, with research applications in chemistry, materials, and biology.
“Vicky also worked at T2 Biosystems, a company that manufactures benchtop spectrometers for analysing human fluid samples using functionalized nanoparticles to identify disease markers. She also had the idea of an NMR tricorder.”
The tricorder idea was investigated further by Blümich’s group, with a student being assigned a feasibility study to design what it would look like as an end-user device for personalized medicine.
Blümich explains, “It would need to be as small as possible, capable of accepting human fluid samples and using lab-on-a-chip chemical analysis. It would feed it into a miniaturized NMR machine about the size of a smartphone, which would house the magnet and all the software. The phone transmits the data over the internet to do the chemometrics analysis and comparison against various databases before sending the information back to the user. It would tell you important information about your nutritional needs, exercise plan, whether you ought to see a doctor, and so on.
“We grew up with fever thermometers and in the age of smartphones these will be replaced by smart analytical devices, which will likely include a magnetic resonance analyzer together with other detectors. NMR has outstanding spectroscopic specificity, and by enhancing the sensitivity using functionalized nanoparticles, you can look at blood and other opaque samples, which other types of spectroscopy cannot do.
“We also need to consider the miniaturization of NMR electronics by Donhee Ham’s group (7). The ability to produce a 2-cm2 chip, including RF amplifiers enables the electronics to disappear into the magnet. Therefore, the dominant size determining component of a compact spectrometer is the magnet – and we are working hard on making that smaller as well!”

Magnets to Market
Andrew Coy, CEO of Magritek, shares his vision of the future.
What is Magritek’s contribution to the field of benchtop NMR?
In the last two years, we introduced a high-performance benchtop NMR spectroscopy system called Spinsolve. It has been very well received in terms of design, performance and capability. Indeed, our industry is going through a very exciting time; we are not the only company selling these instruments, and many people now recognize the benefits that benchtop and portable NMR systems bring.
What does the benchtop market look like today?
Process chemists and chemical engineers are using our technology to monitor reactions in real time using continuous flow NMR operation. These customers come from a range of industries from pharmaceutical to petrochemical to polymers. We also supply our machines to universities and contract research organizations for research and education applications. Some of the new and emerging sectors are in the food industry using NMR spectroscopy to detect adulteration from a quality control perspective.
One of the most recent and compelling benchtop NMR stories is that of Lee Cronin’s group at the University of Glasgow. He took the concept of self-optimized flow systems with in-line analytical monitoring and extended it so that multinuclear and 2D NMR can be performed in the fume hood (1).
What direction is compact NMR moving?
As the field develops, we think NMR will become a far more common analytical measurement technique. Instead of centralized facilities there will be NMR available in the lab next to all the other techniques. You no longer need to be an NMR expert to operate the instrument; in fact, we envisage many applications where the customer does not need to see a spectrum and just gets a result that is useful and valuable. I believe we will see NMR being deployed in a range of environments and applications where people previously considered it impossible.
Where will we be in 10 years?
Over the next five to 10 years, we expect to see benchtop NMR spectrometers appearing in a large percentage of chemistry labs alongside other analytical instruments. We’ll also see new designs that are more robust so that they can be placed in production environments for process control applications, although we expect it will take longer to develop these markets.
NMR spectroscopy is one of the most useful and information rich spectroscopy techniques available to a chemist today. However, the traditional cost, size and fragility of the instruments has meant they are only available in centralized facilities and access is tightly controlled and managed. We think benchtop NMR will enable more people to benefit from the technique, and in turn, funding for research and development in NMR is likely to increase as the value and utility of the technique increases.
Reference
- L. Cronin et al, Chem. Sci., 2015, DOI: 10.1039/c4sc03075c
- Purcell group, Harvard University; Bloch group, Stanford University. Edward Mills Purcell and Felix Bloch shared the 1952 Nobel Prize in Physics for their discoveries.
- R. Whitworth, “NMR Seeds of the Future”, The Analytical Scientist, September 23, (2014). theanalyticalscientist.com/issues/0914/nmr-seeds-of-the-future/
- B. Blümich, “The Incredible Shrinking Scanner: MRI-like Machine Becomes Portable”, [Preview] A portable version of a room-size nuclear magnetic resonance machine can probe the chemistry and structure of objects ranging from mummies to tires, Scientific American, Oct 3, (2008).
- E. Danieli et al., “Small Magnets for Portable NMR Spectrometers”, Angew. Chem. Int. Ed., 49: 4133–4135 (2010). DOI: 10.1002/anie.201000221
- K. Halbach, “Design of Permanent Multipole Magnets with Oriented Rare Earth Cobalt Material”, Nuclear Instruments and Methods 169 (1): 1–10 (1980). DOI:10.1016/0029-554X(80)90094-4. ISSN 0029-554X.
- W.F. Libby, “Atmospheric Helium Three and Radiocarbon from Cosmic Radiation”, Physics Review 69: 671–672 (1946). Bibcode:1946PhRv...69..671L. DOI:10.1103/PhysRev.69.671.2.
- D. Ha et al, “Scalable NMR Spectroscopy with Semiconductor Chips”, PNAS 111 (33) 11955-11960 (2014). DOI: 10.1073/pnas.140201511
Iestyn Armstrong-Smith is special projects editor at Texere Publishing. For around 20 years, he’s been reporting on the impact of industry on the environment and how technology and processes can help bring about beneficial changes. His first editorial post was assistant editor, followed by a promotion to associate editor on LC-GC International. Since then, Iestyn has held senior editorial and management roles for a specialist commercial, scientific, technical marketing and communications company that focuses on the gas, oil and petrochemical industries; a publishing house for market-leading industrial IT magazines; and an educational publisher. “For the last seven years, I ran my own freelance writing business,” says Iestyn.