Introduction
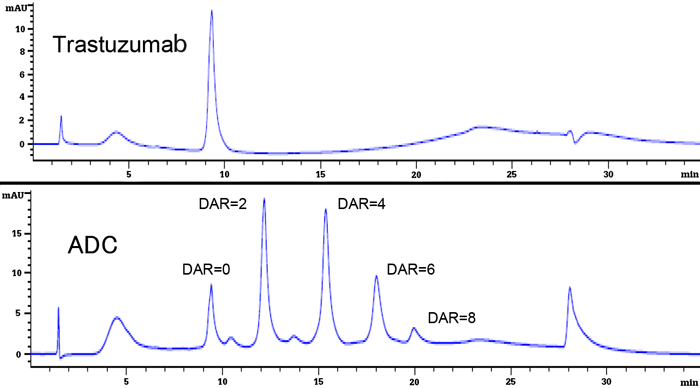
The antibody drug market has continued to expand in recent years, and antibody drugs held 6 of the top 10 blockbuster drug spots for 2015. The most promising antibody drug candidates for next-generation biopharmaceuticals are ADCs (antibody-drug conjugates). ADCs have a structure in which a cytotoxic drug is chemically bonded to an antibody (IgG).