Three mechanisms of polymer degradation have been known as enzymatic, hydrolytic and oxidative. The oxidative degradation is caused by free radicals that are formed by the ultraviolet radiation from the sun at elevated temperature. These free radicals lead to bond cleavage in the polymer chains in the presence of oxygen. Traditional degradation study takes from hours to days to complete due to limited light intensity. CDS’s Photoprobe uses free-space focusing technology improving the light intensity to 800 mW/mm2 within 260–400 nm irradiation wavelength, which reduces the time on weather-induced degradation study down to minutes.
A CDS 6200 Pyroprobe equipped with Drop-In-Sample Chamber (DISC) and Photoprobe was used, and an autosampler module was installed to automate the sequence. High Density Polyethylene (HDPE) of 120 µg was irradiated in the DISC with the presence of air as a reactant gas. The volatiles generated from the photo-reaction were trapped on the analytical trap, and then desorbed to the GC/MS after the photo reaction is completed. Evolved gas analysis was also performed on the original and irradiated HDPE to observe how it had changed. A DISC quartz tube was used as the sample vessel.
Method 1: Weathering
Pyroprobe
DISC Chamber: 60°C
Photoprobe:
UV irradiation: 30min
Reactant Gas: Air 25mL/min
Trap Rest: 40°C
Trap Final: 300°C 3min
Interface: 300°C
Transfer Line: 350°C
Valve Oven: 350°C
GC-MS
Column: 5% phenyl (30m x 0.25mm)
Carrier: Helium, 1.00mL/
min 20:1 split
Injector: 320°C
Oven: 40°C for 2 minutes
12°C/min to 320°C
Mass Range: 35–600amu
Method 2: EGA
DISC Chamber:
Initial: 100°C
Final: 800°C
Ramp: 100°C per min
Interface: 300°C
Valve Oven: 300°C
GC-MS
Column: none: fused silica 1m
Carrier: Helium, 80:1 split
Column Flow: 1.25mL/min
Injector: 300°C
Oven: Isothermal 300°C
Mass Range: 35–600amu
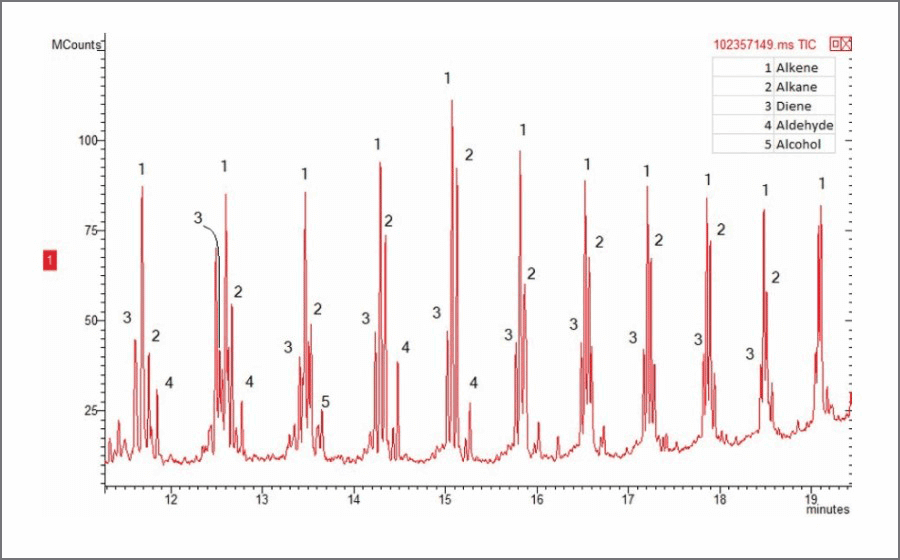
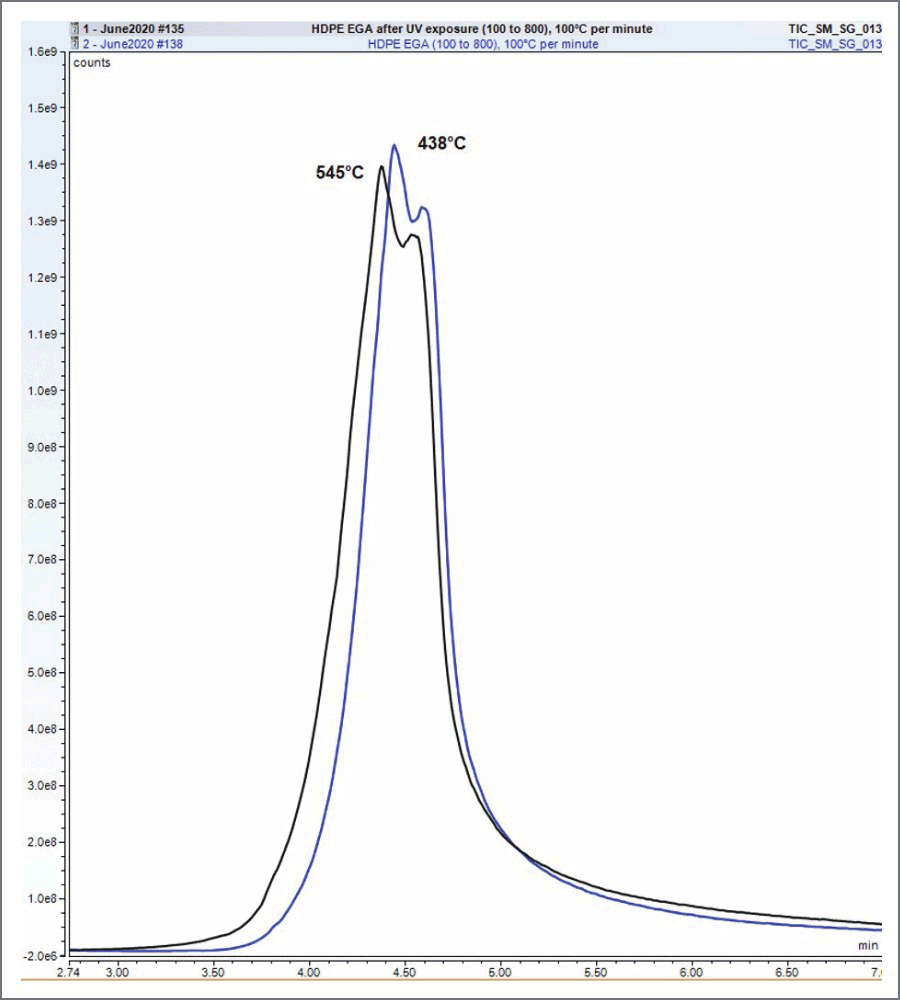
HDPE under UV irradiation and an air atmosphere at a 60°C setpoint produced triplicate peaks associated with random scission of the polymer backbone. Small amounts of aldehydes and alcohols are also present, shown in Figure 1. Near UV light has enough energy to break most common chemical bonds, like the C-C bonds found in HDPE. Once this occurs, free radicals are formed, which can form hydroperoxides (R-O-O·) when oxygen from the atmosphere, is added to the free radical. This radical grabs a hydrogen to form a hydroperoxide (R-O-O-H), which can decompose to form an alkoxy radical (R-O·). The alkoxy radical can stabilize by forming a double bond with beta scission, creating an aldehyde (R=O), or by abstracting a hydrogen to form an alcohol (R-OH). When photo-oxidated HDPE is compared to unaltered HDPE using Evolved Gas Analysis (EGA), the peak attributed to random scission of the polymer backbone has decreased by 7°C indicating that its thermal decomposition temperature has decreased by the same amount.
In addition to analytical pyrolysis, the Photoprobe, the newest addition to the Pyroprobe, can perform online weathering studies with hours of time saved in the UV irradiation step.





