To demonstrate the ability of high-resolution ion mobility (HRIM) to enhance existing chromatographic methods and simplify complex sample analysis.
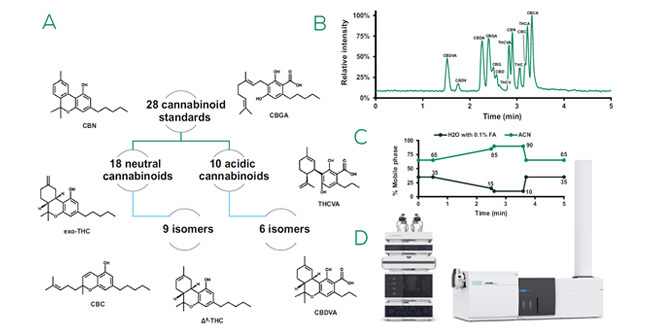
The ongoing quest for sustainable and effective therapeutic agents has increasingly pointed researchers towards the vast pharmacopeia of natural extracts.1 The development of medicines from natural extracts presents a myriad of challenges, beginning with the complex task of novel compound identification. Owing to the immense chemical diversity and complexity within these extracts, pinpointing bioactive compounds, let alone characterizing their precise structures and biological functions, can be akin to finding a needle in a biochemical haystack. Analytical challenges, such as the difficulty of isolating individual compounds in sufficient quantities for testing and the lack of authentic standards for comparative purposes, further complicate the development process, raising the bar for accurate and reliable therapeutic development from natural sources. The presence of isomers — molecules with identical molecular formulas but different structural or spatial arrangements — in natural extracts adds an additional layer of complexity as isomers can significantly impact bioactivity and compound identification efforts. As a representative example to demonstrate the utility of high-resolution ion mobility, we analyzed 28 cannabinoids found in Cannabis sativa, an herbaceous species known to offer a rich tapestry of possibilities, encompassing both psychoactive and non- psychoactive agents with potential applications spanning from pain management to neuroprotection.





