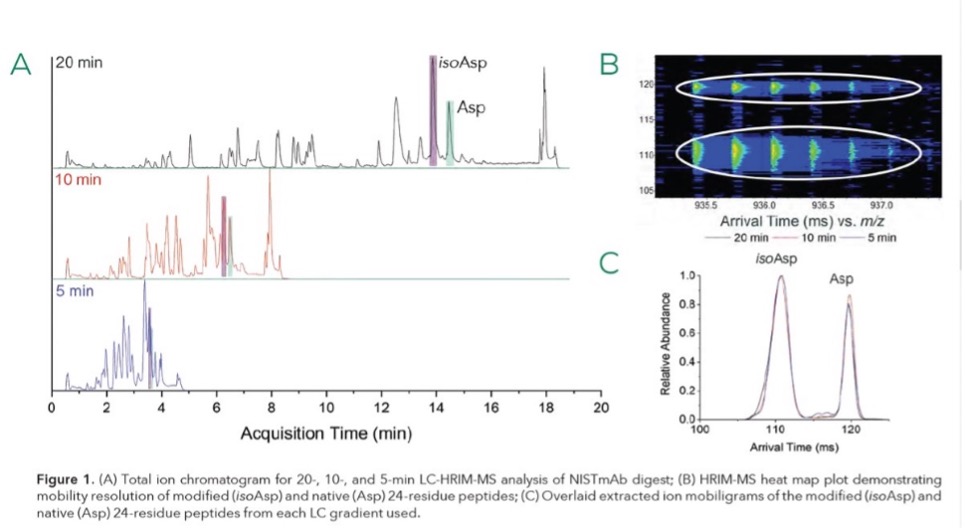
Demonstrate the benefit of incorporating of HRIM into traditional LC-MS workflows for reproducible high throughput targeted PTM analysis for biopharmaceutical characterization.
Post-translational modifications (PTMs) to peptides can disrupt secondary and tertiary protein structure leading to changes in safety or efficacy of protein-based therapeutics. Monitoring of PTMs to ensure production and distribution of safe and efficacious biotherapeutics requires robust and high-throughput analytical techniques capable of detecting extremely small differences in structure. Traditional Liquid Chromatography-Mass Spectrometry (LC-MS) peptide mapping workflows are time consuming and complex, resulting in delayed project turnaround timelines (TATs). We implemented HRIM technology into traditional LC-MS peptide mapping characterization workflows to perform rapid, targeted analysis of Critical Quality Attributes (CQAs) that are often difficult to separate or potentially go undetected with platform LC-MS methods.





