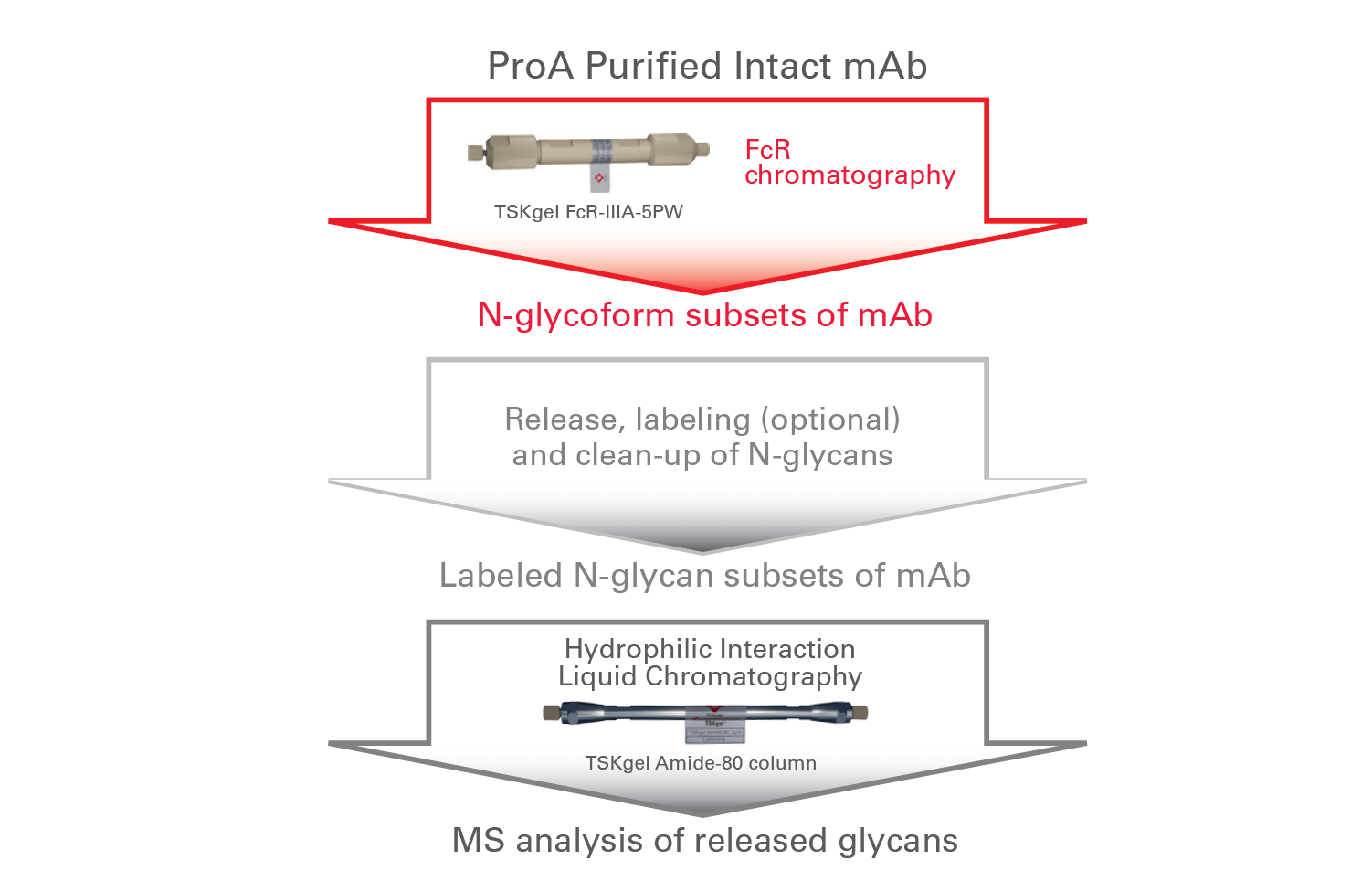
Combining FcR-IIIA affinity separation with HILIC-MS analysis allows rapid screening of antibodies. HILIC-MS confirms the presence and relative quantity of N-glycans in different mAb glycoform fractions for in-depth characterization.
Monoclonal antibodies (mAbs) are an important class of therapeutics with the immense capacity to treat multiple diseases. Due to the complex nature and glycan heterogeneity of these products, characterization and strict control of their critical quality attributes is necessary to maintain product quality and efficacy. The mAb glycans linked to the Asn-297 glycosylation site on the Fc region impact biologic activities such as antibody-dependent cellular cytotoxicity (ADCC) and stability.





