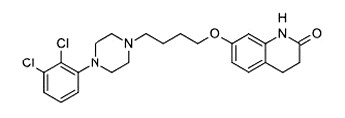
Aripiprazole (USP)
Aripiprazole is an atypical antipsychotic, and it is a partial dopamine agonist.
It is primarily used in the treatment of schizophrenia, bipolar disorder, major depressive disorder, tic disorders, and irritability associated with autism. Common commercial brand names: Abilifyand Aripiprex. Aripiprazole was first approved by the U.S. Food and Drug Administration (FDA) for schizophrenia in November 2002 and the European Medicines Agency in June 2004; for acute manic and mixed episodes associated with bipolar disorder.


We have followed the current Aripiprazole USP monograph (USP38-NF33): Identification –FTIR (197K)
Assay –HPLC (gradient method)
Related Substances -HPLC (gradient method) The HPLC methods are gradient methods, thus non-scalable within allowed changes per chapter 621. The same chromatographic conditions are used for both assay and related substances methods, and a full validation protocol can be found using USP reference standards; USP Aripiprazole RS and USP Aripiprazole Related Compound F RS.