Introduction
Glucagon for Injection (rDNA origin) is a polypeptide hormone identical to human glucagon and is used to treat severe hypoglycemia (low blood sugar).1 It is a single chain polypeptide that contains 29 amino acids residues with a molecular weight of 3483 (Figure 1). As a research tool, accurate quantification of glucagon from biological matrices can help us to better understand diabetes as a function of disease progression and/or drug treatment. Many assays, using different methodologies, exist for glucagon analysis in biological samples.2-7 Glucagon, like other biologics, has historically been quantified using ligand binding assays (LBAs).2-5 With advances in MS and chromatography technologies over the past few years there has been a trend toward the analysis of large molecules by LC-MS/MS. This is in part driven by the fact that LBAs can suffer from significant cross-reactivity issues and lack of standardization. Additionally, LC-MS/MS also has the advantage of shorter development times, greater accuracy and precision, the ability to multiplex, and can readily distinguish between closely related analogues, metabolites or endogenous interferences. Large peptides, such as glucagon, are particularly difficult to analyze by LC-MS/MS as MS sensitivity is low due to poor transfer into the gas phase and poor fragmentation. In addition, glucagon suffers from significant non-specific binding, poor solubility, and must be properly stabilized in biological matrices during collection and sample preparation,6-8 making LC and sample preparation method development challenging.

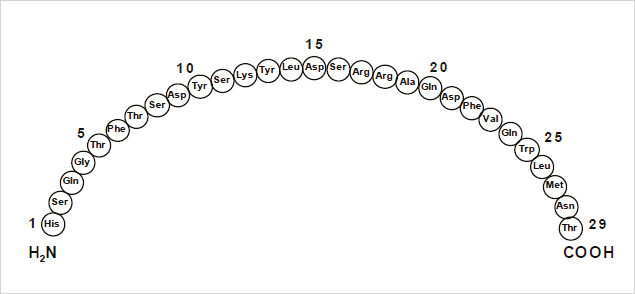 Figure 1: Representative amino acid sequence of glucagon.
Figure 1: Representative amino acid sequence of glucagon.The pharmacokinetic profile of administered exogenous glucagon is characterized by a rapid absorption and elimination with a half-life of <20 minutes, resulting in a total duration of exposure to the peptide of ~2 hours. At the practical clinical dose, 0.25–2.0 ng/mL, maximum glucagon levels of ~8 ng/mL are reached in 20 minutes. Endogenous glucagon in plasma is present in low pg/mL levels (<100 pg/mL), which makes detection by LC-MS/MS even more difficult. The work described herein, uses a combination of selective μElution mixed-mode SPE sample preparation, optimal MS precursor and fragment choice, and the ionKey/MS System (Figure 2) for the highly selective and sensitive quantification of glucagon in human plasma. Detection limits of 12.5 pg/mL using only 200 μL of plasma were achieved with a linear dynamic range from 12.5 to 1,000 pg/mL. This work also capitalizes on the attributes of the ionKey/MS System enabling a 5X reduction in injection volume, a 10X increase in sensitivity, and 4X increase in signal-to-noise (S:N) compared to 2.1 mm I.D. analytical scale method.
 Figure 2. ionKey Source
Figure 2. ionKey Source




