What is peptide mapping?
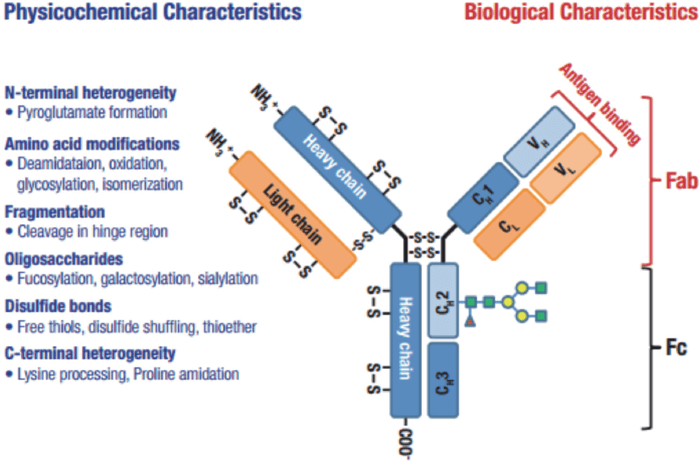
Peptide mapping is a critical workflow in biotherapeutic protein characterization and is essential for elucidating the primary amino acid structure of proteins.
For recombinant protein pharmaceuticals, such as monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), peptide mapping is used for proof of identity, primary structural characterization and quality assurance (QA).

- high levels of irreproducibility
- poor sensitivity
- high levels of time-consuming manual work – with protracted methodologies that are not amenable to automation and often require 24 hours to achieve full protein digestion.