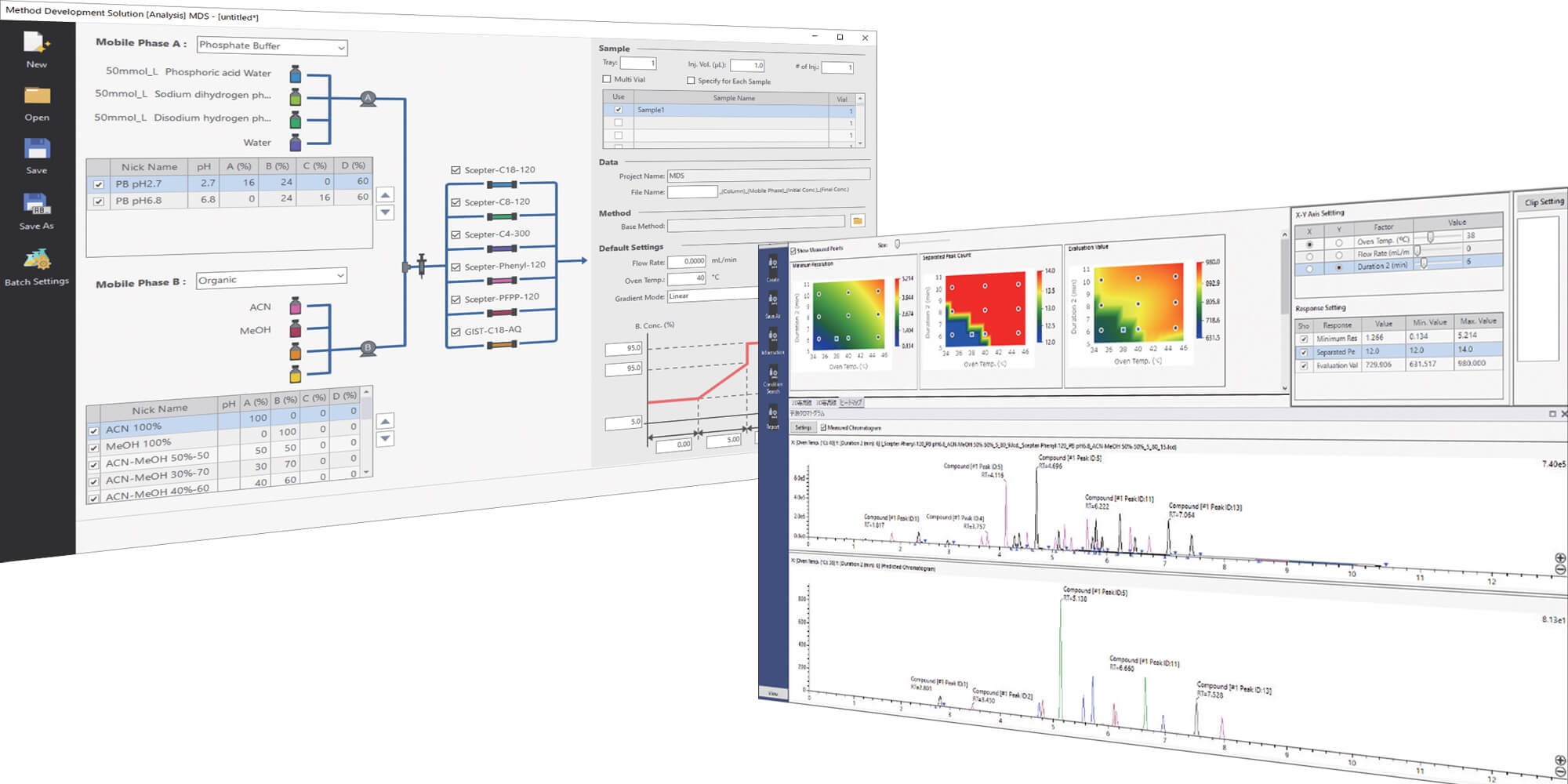
Since pharmaceutical impurities requires strict control to ensure safety, development of highly reliable analysis methods is necessary. LabSolutions MD, a new Shimadzu software for method development, supports efficient method development based on Analytical Quality by Design (AQbD). AQbD-based analysis method development consists of the phases of initial screening, optimization, and robustness evaluation. This article introduces an example of its use in optimization and robustness evaluation of the column and mobile phase selected in the initial screening in order to realize high efficiency in the development of a robust LC method for impurities on ketoprofen.





