Electrochemical investigations are a very current topic in research. In recent times, advancement in technology and industry has resulted in a worldwide increase in energy consumption. A future requirement to face this trend is the development of high capacity and low weight rechargeable batteries for energy storage. Therefore, studies of electrolyte systems or electrode surfaces are of great importance for possible further improvements.
Moreover, in other fields such as biochemistry or catalysis, electrochemistry is greatly beneficial, enabl ing access to molecular information – depending on an applied electrochemical potential. For example, the redox-active center in biomolecules (1), the reaction behavior of catalysts, or the formation of carbon oxides during alcohol oxidation.
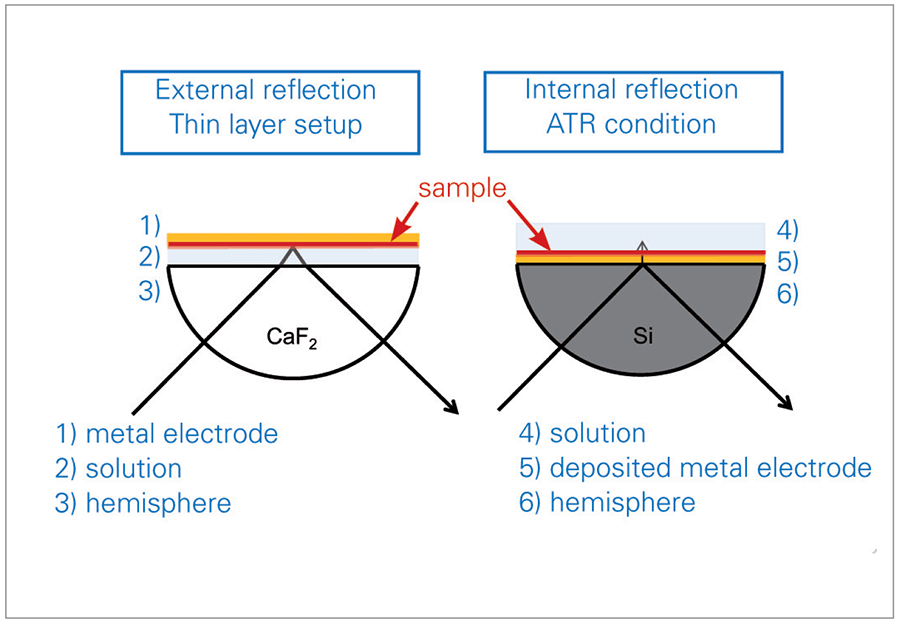
A combination of FTIR spectroscopy with electrochemistry offers insights into the molecular change and reaction processes of studied molecules, in addition to the electrochemical response of the experiment. This valuable method can be applied to investigations of electrolytes or of electrode surfaces. With the BRUKER A530/x reflection unit, prepared for electrochemical cells, both may be studied (2). For an external IR reflectionabsorbance spectroscopy (IRRAS) set-up, a thin layer configuration is used, which allows for the study of the electrolyte and the electrode surface. Alternatively, an internal ref lection ATR set-up can be used to analyze the electrode surface directly with limited influence of the electrolyte (see figure 1).
Overall, the study considered the electrochemical oxidation of a metallic complex, ferrocyanide [Fe(CN)6]4-, with FTIR spectroelectrochemistry. This combined analysis offers the possibility to investigate the molecular change of this compound, depending on the applied electrochemical potential. The oxidation to ferricyanide [Fe(CN)6]3- was illustrated in the form of a SNIFTIRS spectrum and a 3D-plot with the OPUS software. One main advantage of the set-up reported here is that the potentiostat and the spectrometer are connected by a trigger functionality for an allover communication. After setting the experimental parameters and the desired potential procedure, the measurement can be simply started in OPUS and will run automatically. The resulting spectra will be well sorted and assigned to their corresponding potential for subsequent evaluation. In the end, the combination of FTIR spectroscopy and electrochemical investigations makes in situ monitoring of electrochemical processes easy and reliable.
Find out more at www.bruker.com/ INVENIO





