Introduction
Estrogen, androgen, and glucocorticoid metabolism can be used to assess overall hormonal balance during hormone therapy. Urine is the recommended testing matrix for quantification of primary estrogen levels as well as secondary estrogen metabolites when monitoring overall hormone balance, therapy, and detoxification. Non-invasive collection allows for sampling over a 24-hour period, providing insight into a patient’s circadian rhythm. Since many samples are generated for a single patient in any one day, a fast and robust testing protocol is needed for extraction and analysis during clinical testing.
Here, we demonstrate a rapid and reliable sample preparation method using Support Liquid Extraction (SLE+) to extract a suite of 30 hormone analytes from a hydrolyzed urine matrix. Single injection analysis by LC-MS/MS shows that matrix effects are eliminated by the SLE+ protocol and that analyte recovery and sensitivity have excellent clinical utility.

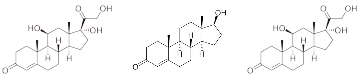 Figure 1. Structures of Estrogen, Androgen, and Glucocorticoid Primary Metabolites
Figure 1. Structures of Estrogen, Androgen, and Glucocorticoid Primary MetabolitesExperimental
Standards were obtained from Steraloids (Newport, RI) and Cerilliant (Round Rock, TX). β-Glucuronidase, ammonium acetate, formic acid, ammonium hydroxide, sodium bicarbonate, dansyl chloride, ammonium formate Sigma-Aldrich (St. Louis, MO). Dichloromethane, ethyl acetate and LC-MS grade solvents were obtained from Fisher Scientific (Waltham, MA). Synthetic Negative Urine was obtained from Golden West Biologicals (Temecula, CA).
Sample Preparation
Hydrolysis: A Tecan Freedom EVO liquid handling system was used to transfer 500 μL of urine, 60 μL internal standard solution, and 600 μL of enzyme-buffer solution (25k units/mL, pH 4.0) to the wells of a 96-well plate. The plate was sealed with an aluminum film and incubated at 38 °C for 16 hours to hydrolyze the glucuronide conjugates of the steroid hormone metabolites.





