Selected ion flow tube mass spectrometry (SIFT-MS) provides rapid, sensitive quantitation of formaldehyde in fragrance samples via headspace analysis. Matrix effects due to the variable headspace compositions of the fragrance mixes were effectively addressed through utilization of the method of standard additions. In this study, three aqueous fragrances were analyzed for formaldehyde content with results ranging from 7 to 85 μg mL-1. The high sample throughput of SIFT-MS means that calibration curves for each matrix are generated rapidly, making the standard additions approach more economic when using SIFT-MS than for chromatographic approaches. This enables SIFT-MS to reduce the time for reporting the first result while increasing the daily throughput. For full standard additions on every sample, three complete analyses (including standards) can be achieved per hour. If the matrix is consistent, then 12 samples can be screened per hour. The ease with which SIFT-MS rapidly analyzes formaldehyde means that it is ideally suited to product quality control – both in the testing laboratory and on the process line.
Volatile compounds can be harmful to human health if present in consumer or drug products at levels that represent a toxicological risk. Formaldehyde is of particular concern, because it is a known human carcinogen (e.g., US NTP (2011), European Commission (2018))) and can arise from impurities in, or degradation of, ingredients, or from residual impurities in packaging materials. Formaldehyde is, however, challenging to analyze using conventional chromatographic techniques (both gas and liquid chromatography; GC and LC) due to its high polarity, high reactivity, and the need for sample preconcentration and solvent extraction. Alternatively, selected ion flow tube mass spectrometry (SIFT-MS) can analyze formaldehyde directly in headspace at toxicologically relevant concentrations.
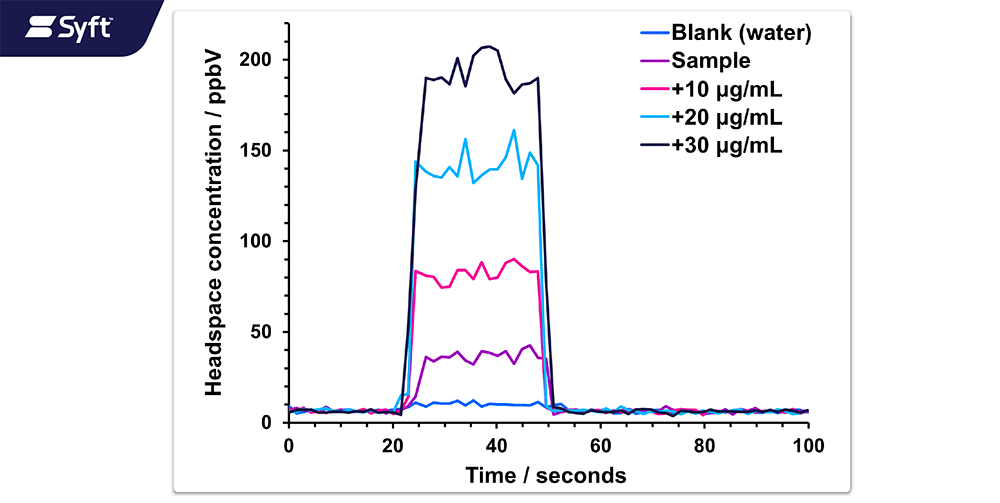
This application note applies SIFT-MS to the analysis of formaldehyde in three fragrance mixes. These mixes have different compositions, but all pose a challenge to SIFT-MS as used conventionally because the total concentration of matrix volatile organic compounds (VOCs) exceeds the dynamic range of the instrument (Smith et al. (2023), Langford (2023)). This complication arises due to the direct, chromatography-free analysis achieved using SIFT-MS, which means that analytes and other matrix volatiles pass through the instrument ionization region simultaneously.





