Water, sediment, and soil are routinely analyzed in the US for extractable petroleum hydrocarbon (EPH) to assess the risk posed by petroleum products in the environment. To obtain an accurate profile of the total EPH, the aliphatic and aromatic hydrocarbons are fractionated and analyzed separately to help identify their health risks. Fractionation of aliphatic and aromatic hydrocarbons is typically carried out according to the Massachusetts Department of Environmental Protection (MADEP) or New Jersey Department of Environmental Protection (NJDEP) protocols using solid-phase extraction (SPE) with heat-treated silica gel [1,2]. One of the biggest problems encountered with this approach is the deactivation of the silica gel due to its hygroscopic nature, which can lead to inconsistent results, low recoveries, and breakthrough of the aromatic fraction into the aliphatic fraction (or vice versa). Furthermore, the volume of n-hexane has to be optimized for each batch of silica cartridges so that only the aliphatic hydrocarbons are eluted without breakthrough of the aromatic hydrocarbons (e.g. naphthalene and 2-methyl naphthalene). This can be a time-consuming and tedious process that is far from ideal for high-throughput testing labs.
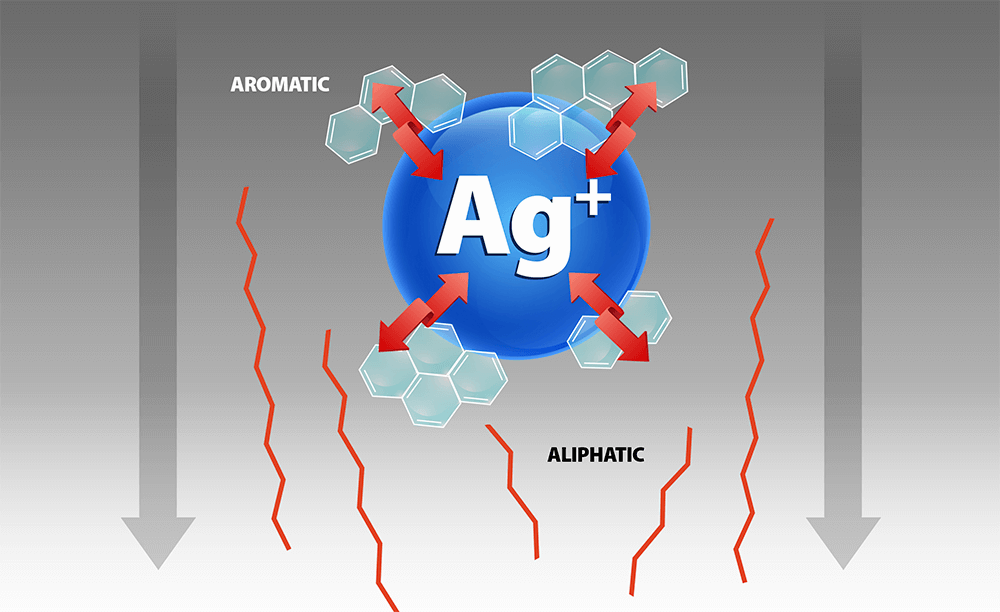
This application note outlines a new specialized SPE sorbent designed to help overcome the challenges associated with traditional silica gel fractionation. The new EPH fractionation sorbent consists of silver ions functionalized onto a solid support. Aromatic hydrocarbons are selectively retained on the sorbent by forming a charge-transfer complex with the silver ions. This ensures high capacity for the aromatic hydrocarbons and no breakthrough into the aliphatic fraction. Consistent lot-to-lot reproducibility without the need to optimize the elution solvent for each batch of cartridges are two of biggest advantages of this new sorbent. In addition, the fractionation protocol is easier, faster, uses less solvent than silica cartridges, while the use of acetone instead of dichloromethane as the elution solvent for the aromatic fraction is significantly more environmentally friendly.