Introduction
Reversed phase chromatography based on traditional silica is the workhorse for the separation and purification of samples in the analytical, drug discovery and development laboratories. The pH range where these traditional stationary phases operate is between 2 and 8. Scientists regularly come across applications where selectivity, resolution as well as loading onto the column are challenging at the above mentioned pH range. Therefore, researchers seek an alternative stationary phase material that resists and has a consistent performance at a wider pH window to effectively and efficiently analyze challenging mixtures as well as isolate compounds outside the common pH range.
Organosilane enforced silica based materials with high mechanical and chemical stability at a wider range of pH constitute an important alternative for scientists to use in separation and purification of samples. This application note illustrates the utility of such enforced phases using pH as a tool. This work also shows that consistent packing of stable materials across different column sizes allows for straight forward scale-up.

Analysis of an alkaloid mixture at low pH
The chromatographic analysis of a sample containing lidocaine, papaverine, noscapine, and diphenhydramine was carried out at low pH using a Kromasil EternityXT 5 μm C18, 4.6 x 150 mm column. The mobile phase composition was acetonitrile/water/formic acid [30/70/0.1] at a flow rate of 1.0 mL/min, temperature 30°C and the run was monitored using a UV detector at 220 nm. The chromatographic result is shown in Figure 1.
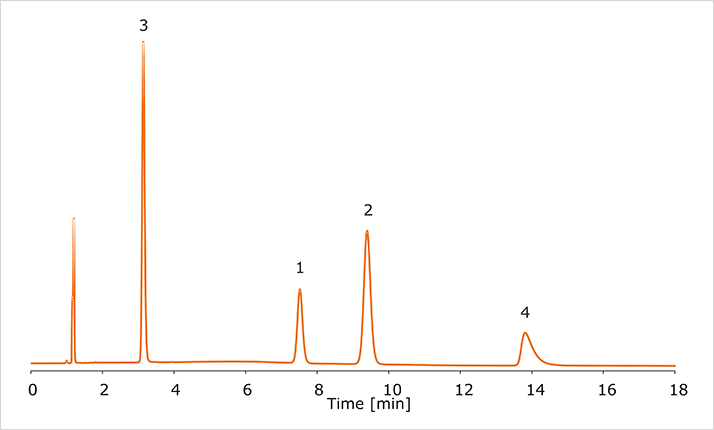 Figure 1: Analysis of a sample at low pH
Figure 1: Analysis of a sample at low pHSubstances: 1: Lidocaine, 2: Papaverine, 3: Noscapine, 4: Diphenhydramine Column: Kromasil EternityXT 5 μm C18, 4.6 x150 mm Mobile phase: acetonitrile/water/formic acid [30/70/0.1] Flow rate: 1.0 mL/min Temperature: 30°C Detection: UV @ 220 nm As seen in the figure, these bases exit the column quickly. The low retention times are due to the nature of the compounds in the sample combined with the low pH conditions resulting in ionized substances that elute fast, compromising selectivity and resolution. Also, if the scientist would need to purify any of the compounds in this mixture, the loading per run would be low due to the low resolution between the peaks, resulting in low productivity and yield per run, contributing to low efficiency in the laboratory.





