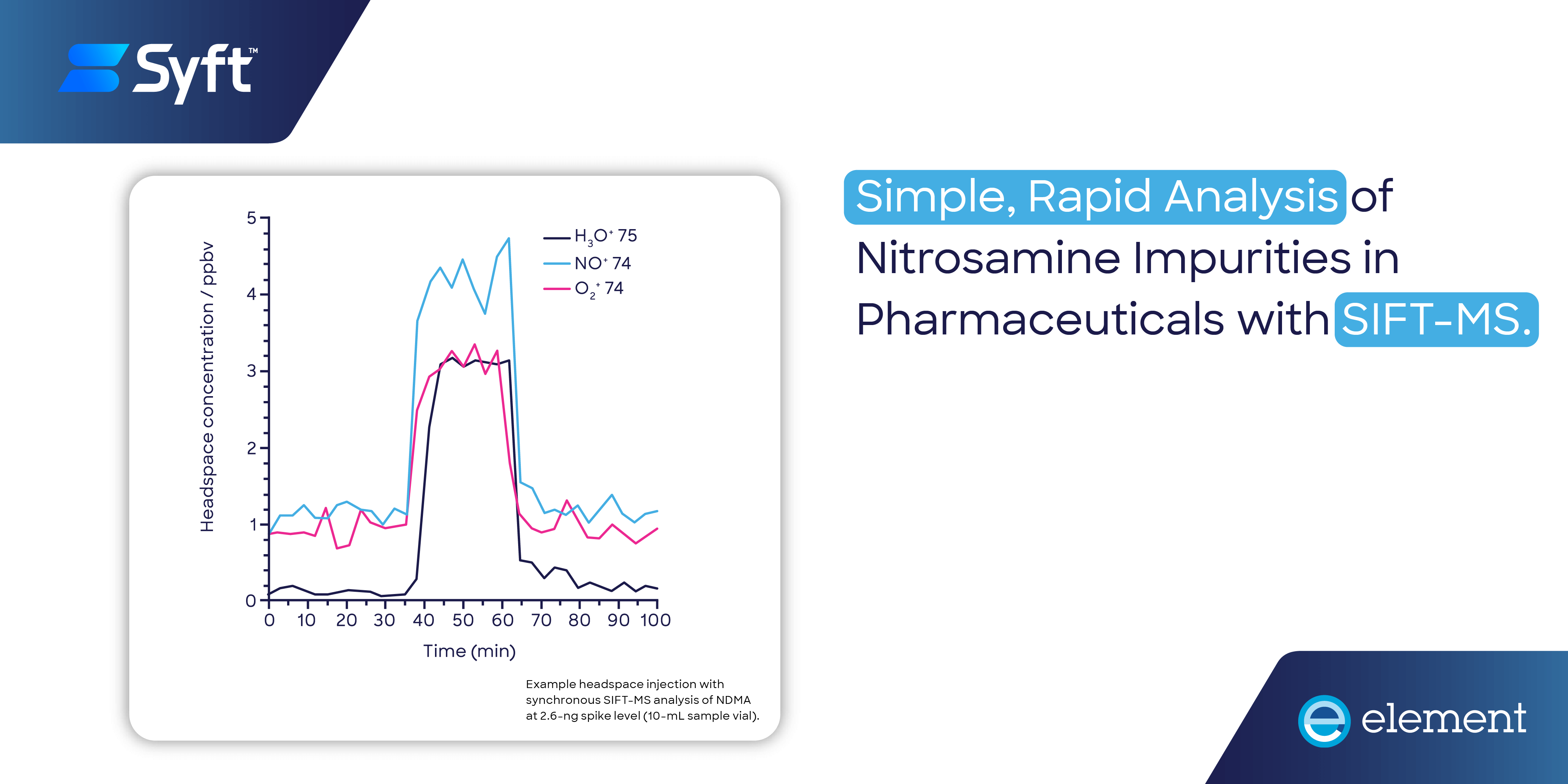
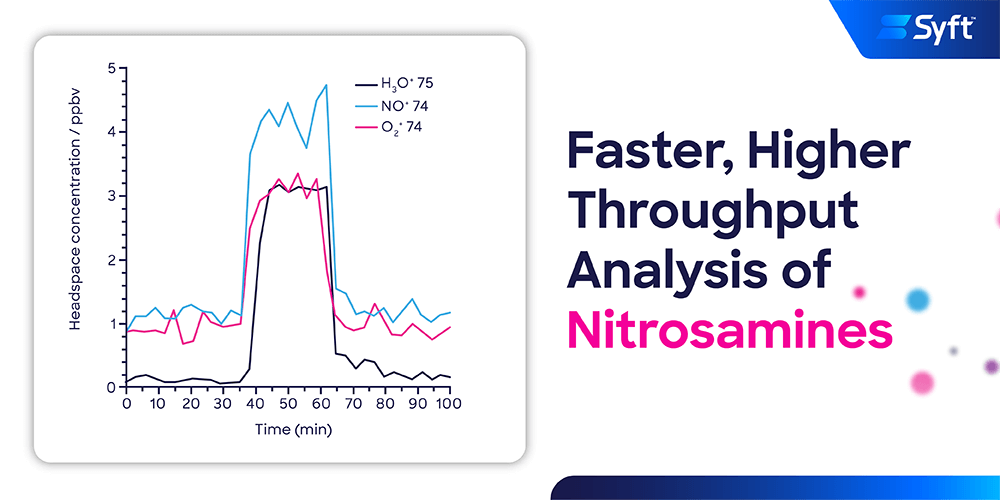
Quantitative analysis of volatile nitrosamine impurities in drug products is greatly simplified using SIFT-MS and has a three-fold throughput advantage (excluding sample prep benefits) over chromatographic methods.
Mutagenic N-nitrosamine impurities are found at trace concentrations in certain pharmaceutical products as byproducts of synthesis or, less commonly, through migration from packaging materials. They are traditionally analyzed using chromatographic techniques that require significant sample preparation and have relatively low sample throughputs. SIFT-MS simplifies and speeds up analysis of trace volatile N-nitrosamine impurities, with a throughput of 12 samples/hr (three times faster than gas and liquid chromatography methods assuming they utilize fully automated sample preparation) and only 70 minutes to first result (including full calibration set; over twice as fast as chromatographic methods). This application note describes headspace-SIFT-MS analysis of ranitidine products and achieves a limit of quantitation of 2 ng g-1 for NDMA in 500 mg of drug product.
The known or suspected mutagenicity of many N-nitrosamines – in particular, N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) – means that their presence in any product to which humans are exposed is of concern. Despite the occurrence of these compounds being well-known in water, beverages, and foods, their discovery in sartan medicines in 2018 came as something of a shock to the pharmaceutical industry (Golob et al. (2022) and references therein). Elastomeric sources of N-nitrosamines – as used in various packaging components – are typically very well controlled due to improvements made in the manufacture of elastomers due to historic issues in that industry (Boltres (2021)). Investigations have revealed that most N-nitrosamine issues arise from nitrosating agents used in synthesis – especially when secondary amines are present (EMA (2021); US FDA (2021)).





