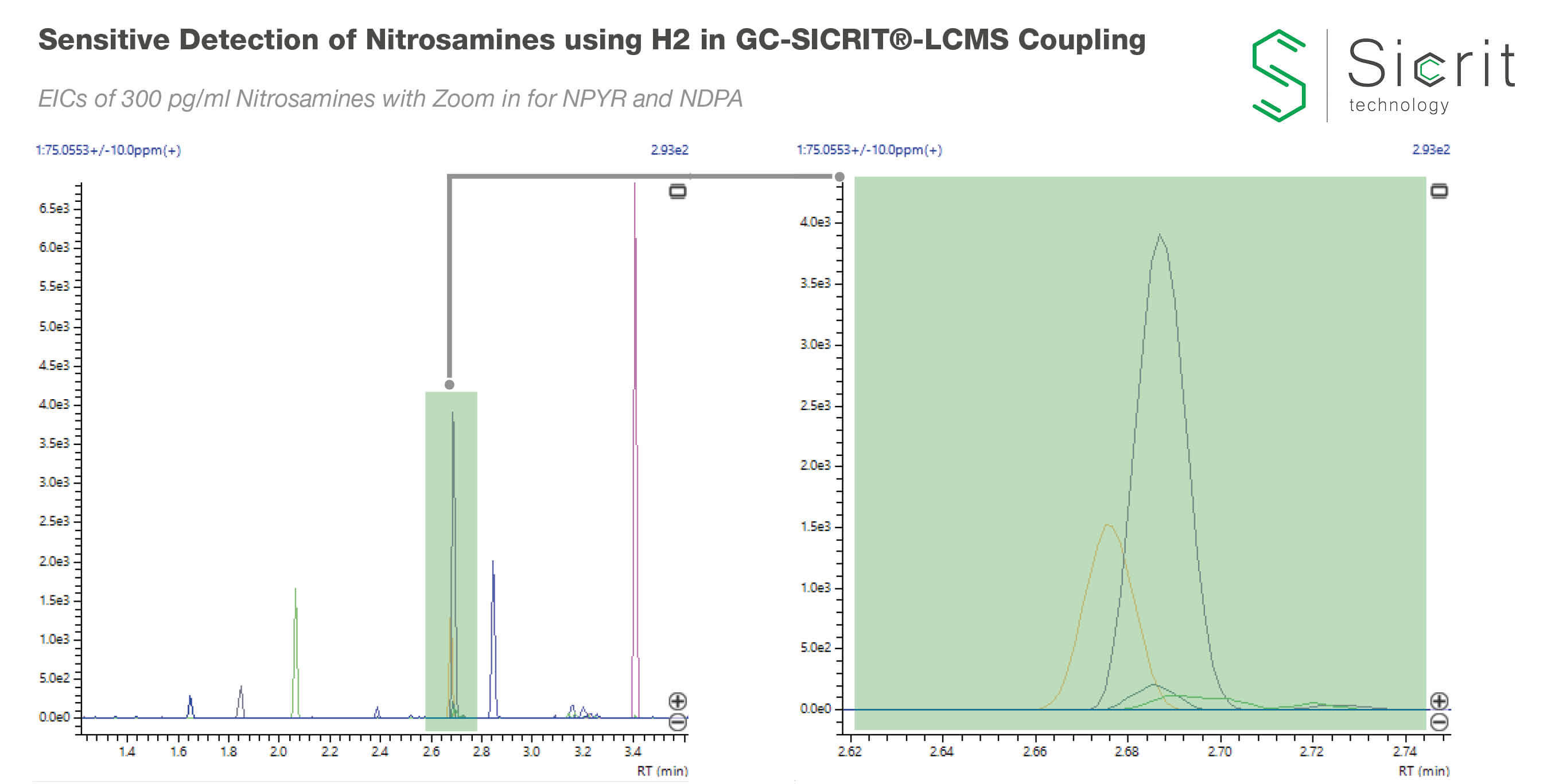
In this study we demonstrate ultra-sensitive determination of various nitrosamine compounds by GC-SICRIT®-MS using H2 as carrier gas and full scan high resolution MS data from Shimadzu LCMS-9030. Compared to standard GC-EI-MS this combination enables a reduction in GC run times by 50% with no loss of sensitivity and the capability for parallel non target quality control. The setup was tested against real world pharmaceutical samples (dissolved tablet) spiked with nitrosamines at low pg levels.
Introduction
Cancerogenic nitrosamine impurities are of big concern in pharmaceutical quality control. In 2018, FDA and EMA initiated a recall of Valsartan products of different manufacturers due to NDMA (N-nitrosodimethylamine) impurities and recommended review of manufacturing processes. Recently, both authorities prompted respective actions also for Ranitidine drugs. The occurrence of nitrosamines in drugs cannot be only attributed to byproducts in raw materials, but also can be formed during the processing stage. Thus, the whole manufacturing processes must be investigated. As the recalls are associated with serious financial losses, there is a great manufacturer’s need of analytical methods for tracing back nitrosamines in their quality assurance.
However, looking at the current analytical techniques, there are challenges in nitrosamine determination for pharma quality control applications. Hence pharmaceutical quality control and drug purity is mainly done by LC-MS/MS analysis, it would be obvious to include nitrosamine analysis into routine LC-MS drug purity control workflows. It has been shown that conventional LC-MS ionization techniques as ESI and APCI are limited in their ionization potential for simultaneous analysis of nitrosamines and the active drug.
In this study we show that GC coupling to (LC-)MS instruments using SICRIT® and its broad ionization range can overcome these shortcomings and presents a well-suited method for simultaneous determination of nitrosamine compounds and active ingredients at very high sensitivities.





