Simplify Raw Material Identification with Through-Container Analysis
Spatially offset Raman Spectroscopy (SORS) eliminates sampling from individual containers resulting in time and cost savings
SORS enables the optical probing of analytes through light-obscuring barriers for non-invasive chemical characterization. The Agilent Vaya Raman raw material identity verification system is a handheld instrument that utilizes this technology and accelerates quality control testing in the pharmaceutical and biopharmaceutical industries. Vaya verifies raw material identity through unopened transparent and non-transparent packaging, testing more containers for the same cost by reducing the need for sampling. Incoming goods can be tested quickly in the warehouse on receipt, reducing operator time and sample-handling booth usage. Testing through sealed containers also avoids handler exposure to high-potency APIs and maintains the shelf life of sterile contents, helping to prevent unnecessary waste.
Solutions for Accelerated Raw Materials Identification. Reducing the turnaround time and cost of pharmaceutical raw material quality control.

Raw material identification (RMID) in a GMP environment is mandated to verify incoming materials to ensure the finished product's quality. What if you could reduce the time taken for receipt to a matter of hours rather than days, reducing the cost of testing? Now, you can enable identity verification through transparent/non-transparent containers in the quarantine area.
DownloadRapid Testing of Solvents Through Amber Bottles Using an Agilent Vaya Handheld Raman Spectrometer.

This study used an Agilent Vaya Raman spectrometer with SORS technology to verify and differentiate solvents in amber bottles. The solvents are often used during the analytical analysis of biologics such as mAbs and for the synthesis of oligonucleotides. Vaya can perform a qualitative test directly through amber bottles within a few seconds. The non-invasive method allows an operator to receive, test, and release large batches of raw materials quickly and conveniently.
DownloadRapid Identification of Raw Materials Inside Packaging
Effective RMID verification equipment must be selective and dependable. Testing materials without removing samples from packaging is valuable to pharmaceutical companies, saving time and money and reducing the risk of contamination. The Agilent Vaya Raman system is a SORS handheld spectrometer that identifies raw materials through transparent and opaque containers to simplify and accelerate the receipt of raw materials in cGMP facilities. The Vaya was used in this study to differentiate closely related materials. The analytical procedure guidelines for identity testing were followed in ICH Q2 (R1).
DownloadIdentification of Commercially Available Oligonucleotide Starting Materials Directly Through Containers
Handheld Raman spectrometers can selectively identify oligonucleotide starting materials, also known as phosphoramidites, directly through amber bottles. This application note discusses the theory and deployment of Agilent handheld Raman systems based on spatially offset Raman spectroscopy (SORS) for chemical ID verification of phosphoramidites in a good manufacturing practice (GMP) environment.
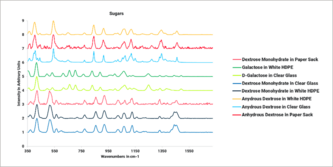
DownloadIdentifying Raw Materials Directly Through Paper Sacks

Paper sacks are often used as containers for raw materials for manufacturing pharmaceutical products. Their enduring popularity continues, as they can easily be disposed of or recycled to minimize environmental impact. They are also the most cost-effective way to package and ship high-volume raw materials. The Agilent Vaya Raman system is a handheld spectrometer capable of identifying raw materials through transparent and opaque containers to simplify and accelerate the receipt of raw materials in GMP environments. This application note highlights how the Vaya instrument can be used to identify raw materials through paper sacks in a pharmaceutical warehouse.
DownloadRapid Identification of Polysorbates 20 and 80 Directly Through Amber Bottles

Under ICH requirements on raw materials, bio-pharmaceutical and pharmaceutical manufacturers must verify the identify of polysorbates before use in Production. Conventional handheld Raman struggles to differentiate polysorbates through amber bottles, which means sampling must be utilized. Spatially Offset Raman spectroscopy enables verification of polysorbates through amber glass, maintaining quality and sterility of raw materials.
Download