Celltrion cited a number of analytical methods in its successful European application for approval for Remsima (a biosimilar to infliximab). The primary structure was assessed using:
- liquid chromatography-mass spectrometry
- (LC-MS) peptide mapping
- LC-MS intact mass measurements
- amino-acid analysis/molar absorptivity studies
- N- and C-terminal sequencing.
- FTIR
- differential scanning calorimetry
- circular dichroism
- free thiol and S–S studies
- antibody arrays
- X-ray crystallographic techniques.
- size exclusion chromatography (SEC)
- SEC with multi-angle light scattering (MALS)
- analytical ultracentrifugation
- capillary electrophoresis-SDS studies.
Things have come on apace since the first biosimilar was given authorization in the EU in 2006. That was Omnitrope, a somatropin, a simple biosimilar product because it was a small non-glycosylated protein. The EU issued its first guidelines in 2005, but SGS M-Scan, as a company, had been working with biosimilar manufacturers prior to that. We were working a little bit ‘blind’ and were treating these molecules as you would any other protein, using the same techniques to interrogate the structure. Now, we have far better and more clearly drafted guidelines, and in addition to overarching directives and guidelines on quality, clinical and non-clinical requirements, there are product- or class-specific guidelines for certain molecules (1). The EU now has over 20 biosimilar products, including monoclonal antibodies (mAbs). Seven or eight years ago, people didn’t think we would ever have biosimilar mAbs – the analytical and clinical challenge appeared too great. Today, we have two products in the EU, etanercept and infliximab from two different manufacturers, and even have approval in the US.
Regulations: getting in on the acts
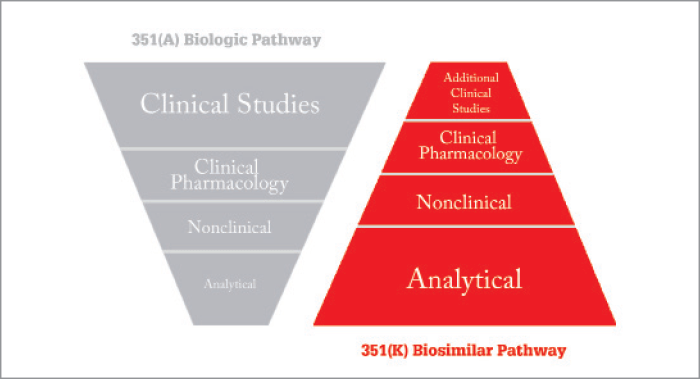
The European market is already being targeted by many non-European manufacturers, and numerous other countries worldwide have published and promoted their own pathways for biosimilars (many have chosen to adopt or adapt the European guidelines). The World Health Organization has also got in on the act by publishing guidelines on the evaluation of similar biotherapeutic products (SBPs) in 2009; supplementing these in March 2016 with a specific draft document on the evaluation of monoclonal antibody SBPs (2, 3). The US was late to enter the biosimilars arena, with the introduction of the Biologics Price Competition and Innovation (BPCI) Act in 2010, which proposed the 351(k) pathway of the Public Health Services Act. It took another two years for the FDA to issue guidance for biosimilar manufacturers wanting to use this pathway, and further time for finalization; biosimilars have only started being licensed in the country within the past year (4, 5). As an accelerated pathway, 351(k) grants access to licensing based on a comparison with a reference product that has been approved via the standard 351(a) pathway. You can’t proceed onwards with either the FDA or EU pathway until you’ve shown biosimilarity at the analytical level and that there are no clinical meaningful differences in terms of safety and efficacy. In fact, at the discretion of the FDA, a full suite of clinical trials may not be required for the biosimilar – as long as similarity to the originator is proven beyond “residual doubt” ( see Figure 1). It has been postulated that within a few years, clinical efforts may be reduced down to one or two PK/PD safety, efficacy and immunogenicity trials, so most of the biosimilarity could be based on detailed analytics. The 351 k pathway of the BPCI Act defines two types of biosimilar: an ordinary biosimilar and an interchangeable biosimilar. An interchangeable product may be substituted for the reference by a pharmacist without the intervention of the prescriber. The wording is something along the lines of: “the interchangeable biosimilar has to be demonstrated to produce the same clinical effect in any given patient”. Right now, as there are no specific guidelines, companies are trying to understand how to demonstrate this. When the EMA authorize a biosimilar, they’re saying the molecule has been demonstrated to be similar to the originator and that it is safe – they are not involved with the interchangeability issue . Each member country then has to decide whether they will allow this. Biosimilar uptake varies country to country, driven by their healthcare system – in some, depending what the biosimilars are, they are now overtaking the originator molecule in terms of market share. One of the countries within Europe which has seen high uptake is Norway. They are so keen on trying to ensure that biosimilars are introduced into the Norwegian hospitals, so the Norwegian Health Agency have decided to conduct a two-year clinical trial funded entirely themselves to look at interchangeability of an infliximab.Establishing “fingerprint-like” biosimilarity
The first step in developing a biosimilar is to determine detailed structural information for the originator molecule, which can then be used as a structural template for the putative biosimilar. It is important that many different batches of the originator are studied, as variation is likely to have occurred over time. The source of the reference product can also be an issue, particularly when developing a biosimilar for a global market, as some countries’ regulators will only permit proof of biosimilarity to a batch from another country if appropriate demonstration is made to show that it is indeed representative of the authorized product in the country of application. The concept of the fingerprint was introduced by the FDA because they wanted a careful consideration of the techniques used to demonstrate comparability. There is little point simply saying you’ll use ten techniques and show some data, if the ten techniques all looked at one parameter or if they didn’t look at interrelated parameters. With fingerprinting, you need to think about the whole molecule; if the molecule is a glycoprotein, then how are these carbohydrates going to interact? Is the shape/conformation of that molecule similar to that of the originator? Developing a fingerprint for a biosimilar involves the use of multiple orthogonal analytical techniques, with appropriate quantitative ranges. By using completely different techniques, you can ensure that there is no bias, carefully building up the fingerprint to cover all quality attributes – those aspects of the molecule that could or would have an impact on its safety or efficacy.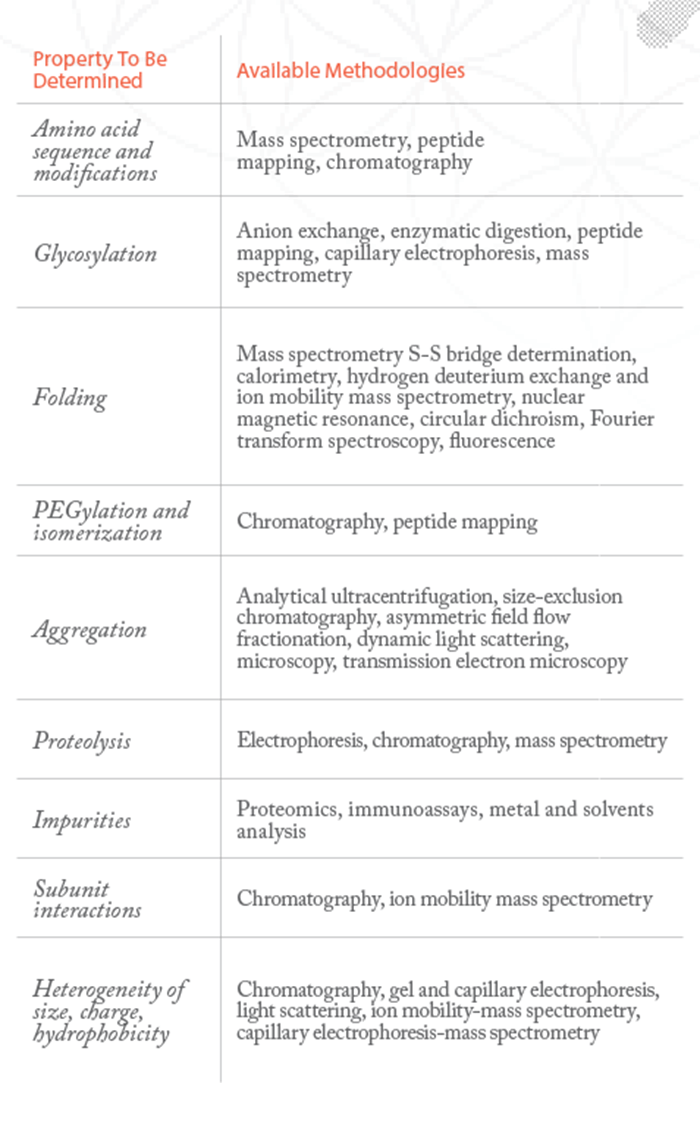
Both clinical and non-clinical data are used to determine similarity. The basis of the biosimilar fingerprint is a statistical approach that demonstrates the two products are analytically similar, but some product attributes are more important than others. Data for the first tier, representing critical quality attributes, should include a statistical equivalence test to prove comparability – and the FDA recommends that these should include those attributes that pose the highest risk when different. A good example is a protein’s glycosylation pattern – the presence of sugars (oligosaccharides) attached to certain amino acid residues – or protein content. Second tier attributes are still important, but less critical, and quality ranges based on standard deviations may be appropriate for these. Those quality attributes in the third tier are the least critical, so graphical or raw data are likely to be sufficient.
The analytical toolkit
The impact of biosimilars over the last ten years has had a hand in driving the analytical sciences forward. As noted, you’ve got to fully understand the originator, down to the last amino acid and the last variant, and that demands excellent analytical tools. I have no doubt that such analytical challenges have been responsible for continuing research in advanced techniques, such as mass spectrometry. And although we already have good mass spectrometry that tells us about the primary protein structure, we need techniques that bridge the information from the protein structure through to its functional activity. One technique that has emerged from an R&D backround in the last four or five years is hydrogen deuterium exchange (HDX) MS. It used to be a tricky technique, but manufacturers worked hard to make it more easily assimilated into the laboratories where it needs to be applied. We’re also seeing techniques filtering through from proteomics, where we can use mass spectrometry, either labelled or non–labelled, to strive for full quantitation, for example, of the host cell proteins that are present in the final products. ICH Topic Q6B is a guideline which lays down test procedures for setting quality specifications for biological drug products. It demands multiple physicochemical and structural analyses, and is an excellent starting point when determining a strategy for proving biosimilarity. Six specification requirements for structural characterization are mentioned:- amino acid sequence
- amino acid composition
- terminal amino acid sequences
- peptide map
- sulfhydryl group(s) and disulfide bridges
- carbohydrate structure (if appropriate).
- molecular weight or size
- isoform pattern
- extinction coefficient
- electrophoretic pattern
- liquid chromatographic pattern
- spectroscopic profiles.
Not so sweet
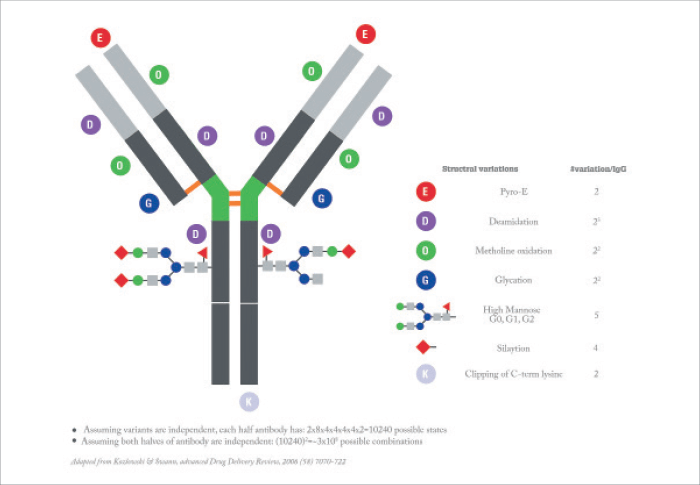
Glycosylation is perhaps one of the most important post-translational modifications (PTM) that occurs during the translation and manufacture of a protein, because it can affect efficacy and, in some cases, result in immunogenicity. Glycosylation modifications cannot be predicted from the gene sequence and have to be determined experimentally. Furthermore, the unpredictable addition of sugars greatly adds to the heterogeneity of the biologic medicine. As an example, just one immunoglobulin G-type molecule has been estimated to have 3x108 potential variations (see Figure 2). One technique that can be applied here is electrospray ionization (ESI) MS, which can provide insight into the number and nature of carbohydrates that are attached on both the reference drug and the biosimilar.Higher order structure
Biosimilars require study not just of the primary protein structure, but also the secondary, tertiary and quaternary structure – the way the protein folds and then interacts in the biological matrix. The conformation of the biologic also has a bearing on its activity and is another important area of investigation when developing a fingerprint for biosimilarity. The renewed focus on structure has led to the resurgence of many physicochemical techniques, for example, circular dichroism, field-flow fractionation, and techniques to look at dimerization and aggregation, such as analytical ultracentrifugation.Celltrion cited a number of analytical methods in its successful European application for approval for Remsima (a biosimilar to infliximab). The primary structure was assessed using:
- liquid chromatography-mass spectrometry
- (LC-MS) peptide mapping
- LC-MS intact mass measurements
- amino-acid analysis/molar absorptivity studies
- N- and C-terminal sequencing.
- FTIR
- differential scanning calorimetry
- circular dichroism
- free thiol and S–S studies
- antibody arrays
- X-ray crystallographic techniques.
- size exclusion chromatography (SEC)
- SEC with multi-angle light scattering (MALS)
- analytical ultracentrifugation
- capillary electrophoresis-SDS studies.
Indeed, many techniques – both qualitative and quantitative – can be applied to determine higher order structure. One of the most commonly applied quantitative techniques is circular dichroism, which is sensitive to helix content and provides information about both secondary and tertiary structure. On the down side, the presence of buffers in the formulation can interfere with the results. Fourier transform infrared (FTIR) spectroscopy is another quantitative method for secondary structure determination that is sensitive to sheet content and less likely to be affected by buffers, thus illustrating the need for orthogonal techniques.
Both intrinsic and extrinsic fluorescence techniques can be used – the former for local tertiary structure, and the latter for surface hydrophobicity – but only give qualitative results. Other qualitative methods include differential scanning calorimetry, which looks at thermal stability, and UV-vis spectroscopy for local tertiary structure. As previously noted, a technique that has emerged from research applications is HDX-MS, which highlights details of dynamics, conformation and interactions, but is expensive and has significant data processing requirements. Another technique more normally applied in a research setting is two-dimensional protein nuclear magnetic resonance. The way that biologics oligomerize and aggregate must also be studied. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) is an inexpensive, routine but low-throughput tool for assessing aggregates, and dynamic light scattering (DLS) can be used to look for high-molecular weight aggregates. Oligomers and aggregates can both be investigated using sedimentation velocity analytical ultracentrifugation (SV-AUC) and size-exclusion chromatography with multi-angle light scattering (SEC-MALS), both of which give quantitative results. I hope I’m painting a picture here about the complexity of the task. The biosimilar boom has really driven analytical science to apply new techniques but also utilize techniques that have been around for a while but perhaps needed updating. In fact, we now have about 100 different orthogonal techniques that we can use to look at the structure of a biosimilar in a comparative way.
The science of safety: now and in the future
It is not sufficient merely to assess comparative structure: comparative functional assays also have to be performed. Suitable quantitative biological assays have to be developed and run to link product attributes with biological properties – and the results for the originator and biosimilar must correlate well if similarity is to be accepted by regulators. The assay must also be able to assess properties appropriate to the nature of the biosimilar. Again, a range of techniques can be applied, including biochemical assays, such as ligand binding, immunoassays, enzymatic assays and radioimmunoassay studies. Others techniques are cell-culture based, including cytotoxicity, cell uptake, proliferation, secondary messenger and PCR-based functional assays. The chosen techniques, both structural and functional, will vary from one biosimilar to another. However, the resulting information should always cover a sufficiently wide range of parameters to give regulators confidence that the biosimilar will behave in a similar fashion to its reference product in patients. For an example of how many different techniques may be needed for one product, see “The Analytical Challenge.”Big challenge, big data
A large amount of data is generated, so the crunching of data is a major problem and has to be addressed, either by the manufacturers of the instrumentation or the companies working with them. In either case, it needs to be tackled in a way that is satisfactory to the regulatory authorities. In particular, those authorities expect analysis to be performed in a GMP environment and will require any computerized data interpretation to be fully compliant with GMP. We need to ensure that we have good, robust methods that produce reliable quantitative data so that comparability can be conducted using head-to-head statistical analysis. With the inevitable variability between biologic products manufactured in different cell lines, careful comparative studies are essential if regulators are to be convinced that a biosimilar is both safe and effective. By applying multiple orthogonal analytical techniques to both the reference originator product and the biosimilar, including functional studies, an all-important fingerprint of biosimilarity can give confidence that patients will not be adversely affected if they are prescribed a biosimilar instead of the originator product. The role of analytical science has never been more vital. Fiona Greer is Life Sciences Global Director, Biopharma Services Development at SGS.Fiona Greer, Life Sciences Global Director, Biopharma Services Development at SGS, shares her analytical journey through the biotechnology boom.
How did you get into analytical science?
At school I was always into medicine. I initially wanted to be a surgeon but had second thoughts. I knew I wanted to be involved in science though, and was extremely interested in microbiology, so I went to university to do a degree in Food Science and Microbiology. That was back in the late 1970s – at the start of the biotechnology industry and the use of microbial fermentation. At that point, I got sidetracked into analytical chemistry by an Masters in forensic science, before becoming very interested in analytics and doing a PhD at Aberdeen University in Protein Chemistry. Many years ago, a Bulgarian diplomat was killed with ricin from the castor oil plant. I worked with a similar toxin, a lectin from the kidney bean plant (which is not as potent as ricin; it was very interesting to isolate this toxin and look at its capabilities. I think it was this investigative nature of analytical chemistry that piqued my interest.What was your route into mass spectrometry?
My PhD was spent between Aberdeen University and the Rowett Institute for Nutrition and Health, where they had one of the first gas phase sequencing instruments – a piece of kit that was revolutionizing protein sequencing at the time. Around the same time, Professor Howard Morris, FRS – professor of biochemistry at Imperial College London – was setting up a company (M-Scan) to use mass spectrometry to sequence proteins – pioneering work. I joined Howard’s company in 1984, where we initially used an ionization technique called fast atom bombardment (FAB) to sequence a variety of proteins and glycoproteins from the new biotechnology industry. That was my first foray into applying analytical instrumentation to biotech problems.Sounds like an exciting field...
It was! But actually, protein science was not very trendy at that point – everybody wanted to be a geneticist or molecular biologist. Up until that time – and even during that time – a lot of the scientific focus had been on genetics, working on constructs that could express proteins. It wasn’t until they’d succeeded in engineering and process development that they needed protein science to confirm that the product was the right one. To begin with, we were a small operation, about five or six people in the UK. But by 2010, we had four international sites operating with about 65 people. We had a reputation as the foremost protein and carbohydrate structural lab offering analytical services. At that stage, all four labs were acquired by SGS.Is staying at the cutting edge important to you?
Very much so. In the beginning, we were a very small privately funded company; we had to keep driving forward so we could offer new techniques and capabilities to survive. And it wasn’t just about running the instrumentation – determining the analytical strategies and interpreting the data were also crucial to solving problems. It’s actually a considerable time since I wore a white coat in the lab, but with SGS I’m still focused on pushing forward our capabilities in the laboratories – ensuring that we keep introducing the most up-to-date, properly qualified and validated techniques.What has kept you in the same company for so long?
The interest and excitement. The field has developed rapidly – driven by the challenges we were given by the biotechnology industry. When I first started, we were using a state-of-the-art high-field magnet mass spectrometer made by VG – now Waters – and the largest intact molecule it could look at was probably 6–7,000 Daltons. We had to drive forward both the instrumentation and the ionization techniques to be able to look at intact proteins at high sensitivity and perform MS/MS sequencing. We picked up electrospray very quickly along with MALDI-TOF and Q-TOF instrumentation. Biotechnology is a global industry and I have worked around the world, interacting with a lot of very bright scientists who were setting up companies, trying to exploit their research and bring it through into a commercial product.Why do you think you’ve had such a successful journey?
Sheer bloody-mindedness! Everybody makes their own choices, and maybe I was lucky in that I chose something that I enjoy doing. I get intellectual stimulation from working with very bright people, and it’s scientifically rewarding to look at the new techniques that are coming through and to try and introduce them to the labs that I work with.References
- European Medicines Agency, “Multidisciplinary: biosimilar”. Available at: http://bit.ly/1trteeH. Accessed June 13, 2016. World Health Organization, “Similar biotherapeutic products” (2014). Available at: http://bit.ly/1XiRR8W. Accessed June 13, 2016. World Health Organization, “Guidelines on evaluation of monoclonal antibodies as similar biotherapeutic products (SBPs)”, (2016). Available at: http://bit.ly/1ULeTlr. Accessed June 13, 2016. FDA, “Information for Industry (Biosimilars)”, (2016). Available at: http://1.usa.gov/1wAiQzJ. Accessed June 13, 2016. S Sutton, “The big break for biosimilars?”, The Medicine Maker, 6, PAGE NUMBERS TO BE ADDED (2015). Available at: http://bit.ly/25X2397




