© Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
Monoclonal antibodies (mAbs) represent the largest fraction of licensed, protein-based therapeutics – and for good reason; they have a history of safety and efficacy, and their manufacture is well established. The efficacy of mAb therapeutics is linked to their ability to bind to molecular targets with high specificity and high affinity. Coupling selectivity, size, and the potential to recruit the patient’s own immune system, mAbs represent a modality with therapeutic effects inaccessible to classical small molecule pharmaceuticals.
Further, as the biopharmaceutical industry has enhanced its understanding of mAbs, the development, manufacture, and characterization of novel mAbs has transitioned towards a “platform-based” approach, making their production relatively straightforward and reducing the time-to-market for new therapies.
Complex characterization
As with all drugs, every batch of mAb must have representative samples taken for analysis. The samples must meet specifications that are defined through extensive characterization of the mAb through the clinical trial phases. The release criteria are defined such that the analytical testing ensures quality, safety, and efficacy of the manufactured drug product.
Each batch of mAb contains multitudes – in other words, the drug substance produced actually exists in many different related, but distinct forms. mAbs are large, structurally complicated drugs; thus, analytical methods must be sensitive to their unique chemical, biological, and physical aspects. The mAb’s size (molecular weight), amino acid composition, variations in structure, charge, sugar-based modifications, are just some of the attributes that must be measured to understand the heterogeneity of each batch of mAb. Furthermore, biological activity – including potency and immunological properties – must also be assessed. In short, assurance of the mAb’s identity, purity, and sterility are of utmost importance.
Separation of the overall biologically, chemically, and physically similar isoforms of mAbs from one another – and also from any residual impurities from the manufacturing process – is the main task for analysis. Electrophoretic and chromatographic separation methods are essential tools for the identification and quantification of isoforms and impurities.
Validating a method requires deep understanding of the underlying principle of the technique, the sample, and potential contaminant properties, while controlling for the inherent variability in the instrument, the operators, and even the environment. Controlling these variables with instrument maintenance, effective SOPs, automation for method consistency (well-characterized and controlled reference standards are a must!), and well-trained operators are core to consistently overcoming the challenges of mAb characterization and analysis.
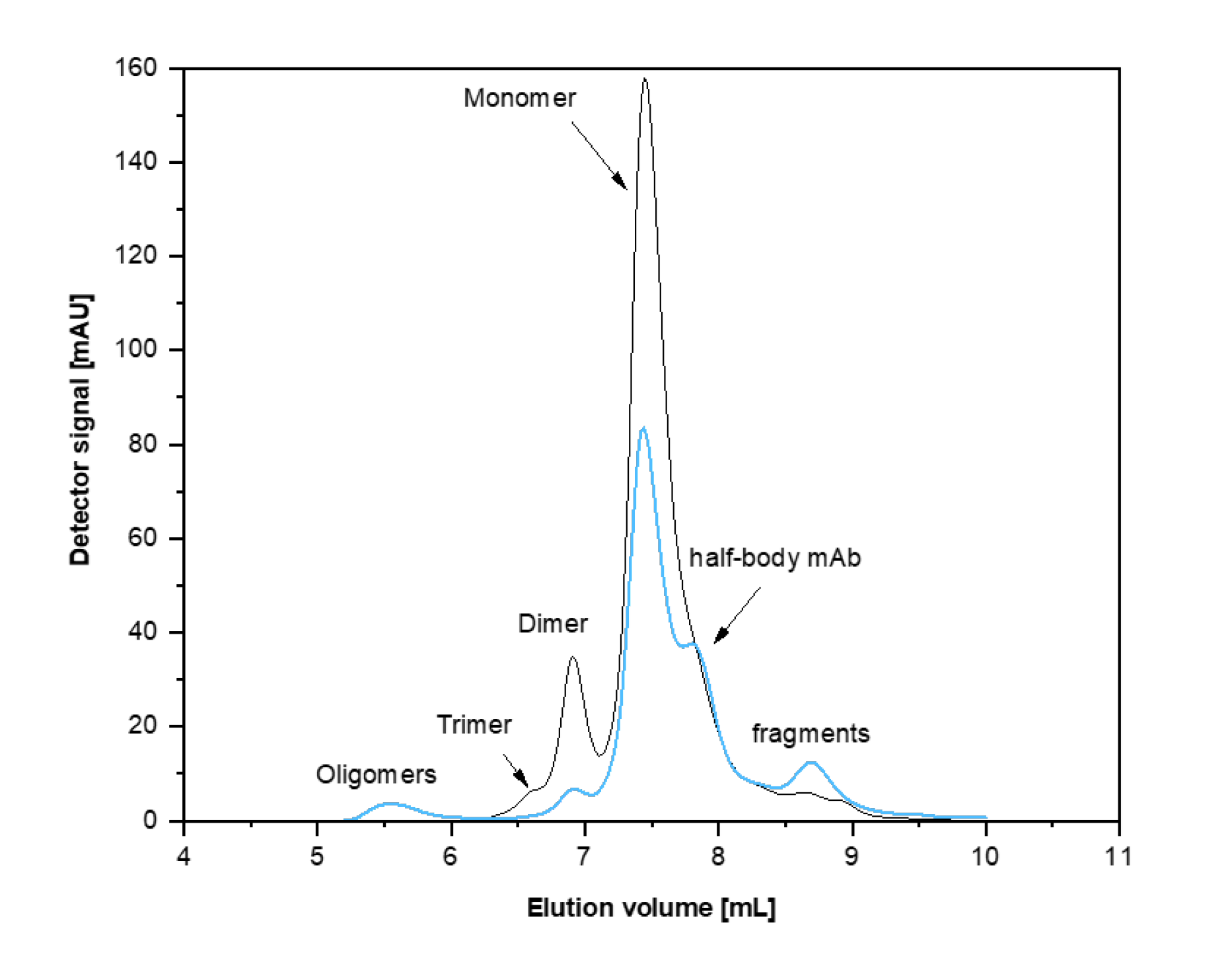
Figure 1. Separation range of the Agilent MAB 3 µm column. The black curve shows the UV signal of a native antibody and its associates plotted against the elution volume. The blue curve is the elugram of fragmented antibody sample by reduction.
© Agilent Technologies, Inc.
Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
Room for improvement?
Increased automation in sample handling, incorporation of process analytical technologies, and implementation of quality by design practices in cGMP can enhance process control and streamline QC testing, potentially reducing the burden on lot release tests. This is contingent on the ability of the mAb manufacturer to support the decision to reduce testing requirements with rigorous scientific studies.
Technology evolves to meet the demands of industry (necessity is the mother of invention); however, the pace at which emerging analytical tools can supersede and displace long-established methodologies is slow. The undertaking required to get a green light is significant, and the efforts of regulatory compliance can be a hurdle to continuous improvement in industry.
Systematic incentivization for regulatory bodies and manufacturers alike to incorporate tools that reduce environmental impacts and streamline manufacturing, while increasing the quality, safety, and efficacy of drugs will be paramount to the continued success of the biopharmaceutical industry.
Taming Complexity
Here, we ask Agilent’s Andreas Mielcarek to share insights from the inside – and to explore the challenges of mAbs through the lens of analytical instrumentation.
Monoclonal antibodies (mAbs) stand at the forefront of biotherapeutics thanks to their targeted efficacy. How does Agilent's portfolio propel mAb therapeutic development?
Agilent's commitment to advancing mAb analytics is exemplified through our meticulously designed portfolio, which addresses key challenges in biotherapeutic development. Our focus on bio-inertness and bio-compatibility sets the stage for tackling various obstacles, including harsh analytical conditions (high salt load and extreme pH values).
By leveraging Agilent’s advanced technology, researchers and developers can overcome common issues, such as sample loss and degradation, to better meet the stringent demands of modern biopharmaceutical analysis. I’d go as far to say that our portfolio empowers the scientists involved in the mAb therapeutic development process by providing robust solutions that enhance efficiency and accuracy – ultimately contributing to the advancement of targeted and effective biotherapeutics.
With mAb analytics growing in complexity, where do you see opportunities for advancements? And how is Agilent contributing in this arena?
The integration of new analytical technologies that enhance efficiency, while meeting regulatory standards is one key area for advancement. Agilent tackles this challenge with a comprehensive suite of analytical tools, including HPLC, MS, UV-Vis, MALS, DLS, automated sample preparation, and so on, that support the industry’s need for precise and comprehensive biopharmaceutical analysis. We’re also focusing on automating analytical methods and incorporating process analytical technologies (PAT) to enable real-time manufacturing control, thus enhancing product quality and consistency.
You mentioned PAT – an increasingly important area. Can you discuss how Agilent’s solutions, particularly online LC, contribute to monitoring critical process parameters (CPP) and critical quality attributes (CQA) in bioprocessing?
Agilent’s Online LC solutions are at the forefront of PAT, offering real-time monitoring of CPP and CQA – foundational metrics for optimizing bioprocessing. This technology delivers continuous analysis during bioproduction, ensuring process consistency and product quality. By integrating our Online LC systems into bioprocessing workflows, manufacturers can achieve tighter control over their processes, enhancing both efficiency and compliance.

With the move towards a “platform-based” approach in the development, manufacture, and characterization of new mAbs, how does Agilent’s suite of solutions support this trend – especially for those just starting in the biopharma industry?
For newcomers to the field, our solutions are designed to reduce complexity while providing high-quality, actionable data. The integration of our systems into the biopharma workflow enhances the efficiency of analytical processes and ensures that even those with minimal training can achieve reliable results. Our Online LC Monitoring Software, for instance, simplifies the operation of the Online LC, enabling easy monitoring and adjustment of critical process parameters. Through advanced, user-friendly solutions, Agilent aims to equip analytical chemists, especially those embarking on their careers, with the tools and knowledge to contribute effectively to the development of safe and effective biopharmaceuticals.
Agilent appears to be fully committed to supporting the biopharmaceutical industry. What’s your “elevator pitch?!”
Agilent is tackling the challenges in mAb analytics with a multi-faceted strategy. Our extensive suite of analytical technologies is crucial for comprehensive mAb analysis. We emphasize automation and PAT to enhance efficiency and quality. Moreover, the quality by design (QbD) approach is central to our strategy, ensuring robust product development from the outset. In terms of regulatory compliance and environmental sustainability, we’re innovating to meet standards while minimizing our environmental footprint. Additionally, Agilent is committed to fostering education and collaboration to advance analytical methodologies in mAb analytics.