
The entrenchment of gas chromato-graphy-high resolution mass spectro-metry (GC-HRMS) as the tool of choice for quantitation of chlorinated dioxins and furans has left some countries without access to adequate analytical infrastructure. How can advances in instrumentation and technology be leveraged to the benefit of low-economy nations?
The disposal of toxic waste is a matter of enormous concern globally. The problem is most acute in the developing world, where legislation is often in place but not adequately regulated or enforced, opening the door to unscrupulous operators. Despite the potential risks, many poor countries are driven to import toxic waste for disposal as a means of raising revenue. The result? Waste disposal that is at best careless and at worst criminally negligent. To exacerbate the problem, the facilities and instrumentation required for the extraction, sample clean-up and determination of persistent organic pollutants (POPs) is frequently not in place. Consequently, there is a lack of coordinated routine capability. For twenty years now, the quantitation of chlorinated dioxins and furans has been governed internationally by US Environmental Protection Agency (EPA) Method 1613 or methods derived from it. This has led to the entrenchment of HRMS as the analytical option of choice. For quantitation at concentration levels under parts per billion (ppb), selected ion monitoring (SIM) is used. In South Africa, there is no established facility capable of routine dioxin measurement using GC-HRMS. While GC-HRMS was the only available technique that could reach the low levels required by EPA 1613 at the time of its publication, the advancement of instrument technology over the last twenty years has given rise to alternative approaches. There is a clear need for methodology that offers simple implementation, fast turn-around, reliability at EPA Method 1613 levels, and cost effectiveness. Notably, in the study below, GC-HRMS comparative analysis was performed overseas after threshold screening. A process that was not only extremely costly but also impractical, giving rise to delays that would be unacceptable in critical projects, for example, assessing bird population decline (1) or large-scale crocodile deaths in Kruger National Park (2).
The System
GC×GC-TOFMS: LECO Pegasus 4D (Agilent GC and autosampler, a secondary oven, and a dual stage modulator). Liquid nitrogen cooling was used for the cold jets; synthetic air for the hot jets. Primary and secondary columns were connected using a press-tight connector. A GC multi-step temperature program was developed to facilitate separation and quantification of the maximum number of POPs that could be present in environmental samples in a single analysis, thus fully benefitting from the added selectivity of GC×GC and the full range mass spectra generated by TOFMS. Tuning: The less conventional 414 ion was used in an attempt to improve the signal intensity in the higher mass range (3). Software: All instrument functions and data processing were managed with LECO ChromaTOF software, including the manual review of all peak identifications and integrations. Searches were performed using a dioxin/ furan user library compiled from the 17 toxic dioxin and furans congener standards.We chose to combine two screening techniques: the H4IIE-luc bioassay and comprehensive GC-time-of-flight MS (GC×GC-TOFMS). The former is fast, user-friendly and inexpensive; the latter is unique in its ability to detect all compounds in complex samples at the levels needed for priority pollutant determination in a single analysis, can provide quantitative data, and is capable of reaching the levels stipulated in EPA Method 1613. Positive samples can also be sent for quantitative analysis using the reference GC-HRMS technique, which removes the costs associated in analyzing negative samples. During quantitative method development, we compared the results of GC×GC-TOFMS with those obtained for the same sample set using GC-HRMS to confirm validity, making it the most comprehensive environmental screening and dioxin determination study to date in South Africa using GC×GC-TOFMS.
In this bioassay, dioxin-like chemicals bind the aryl-hydrocarbon receptor (AhR), initiating transcription of the reporter gene, luciferase. Luciferase activity, proportional to the amount of dioxin-like chemical present, is measured using a microplate-scanning luminometer. This provides information on the cumulative biological effects of the sample, and allows samples to be ranked according to their toxic potential. It is not compound specific, however, reacting with any chemical that can bind to the AhR receptor; it therefore provides no information on the concentration of individual compounds.
GC×GC-TOFMS
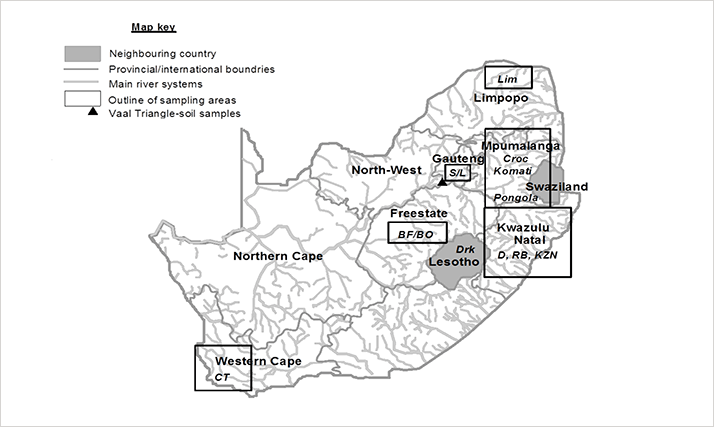
GC×GC-TOFMS perfectly complements the bioassay with the necessary selectivity (through the added peak capacity of GC×GC), and sensitivity (through the focusing effect of the modulator). The instrumentation is more affordable and more user friendly than GC-HRMS. And, because it is not a target molecule technique, GC×GC-TOFMS can provide a comprehensive snapshot of total sample toxic potential in one run (3). Furthermore, it meets the requirements of EPA 1613 by providing acceptable quantitation at the mandated levels of detection (4,5).Fresh soil and sediment samples were taken from diverse regions of South Africa to represent different land-uses and anthropogenic impacts (see Figure 1a). The samples were first analyzed for dioxins and furans by GC-HRMS and then by GC×GC-TOFMS (Table 1). The excellent agreement between GC-HRMS and GC×GC-TOFMS for the total sample toxicity results (total toxic equivalents, TEQ) confirms the reliability of the new method.
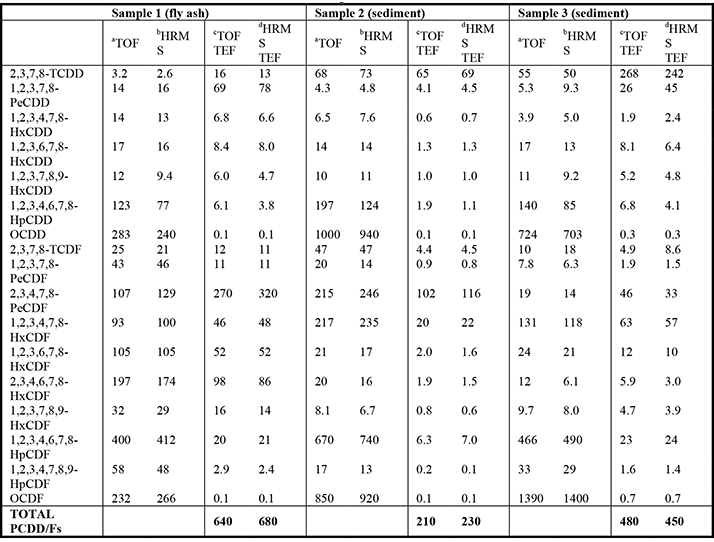
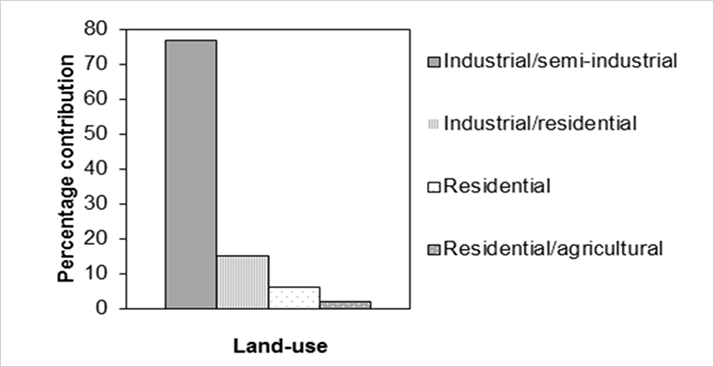
The extraction and clean-up procedure for the H4IIE-luc bioassay and GC×GC-TOFMS samples is similar. Samples are air dried and homogenized. A 40 g portion is mixed with sodium sulfate before accelerated solvent extraction using dichloromethane and hexane. The extracts are treated with activated copper to remove sulfur, and cleaned by gel permeation chromatography (GPC) and acid digestion. The samples for GC×GC-TOFMS analysis are spiked at appropriate levels using the internal standards for the 17 toxic dioxin and furan congeners recommended by EPA 1613. The bioassay detected dioxin-like activity in 22 percent of the sediment samples (BEQ20; n=96) and 58 percent of the soil samples (BEQ20; n=66). BEQ20 indicates a sample extract that gave a 20 percent response to the dioxin (TCDD) positive control. The distribution of dioxin-like activity from different collection sites is shown in Figure 1b. All samples that tested positive and six samples that tested negative in the bioassay analysis were then analyzed by GC×GC-TOFMS for dioxins and furans. Of these, 23 percent of positive soil samples and 41 percent of positive sediment samples were found to be false positives (the bioassay is not dioxin/furan specific). In South Africa, polynuclear aromatic hydrocarbons (PAHs) are generally present at levels an order of magnitude higher than the dioxins/ furans, and this is most likely the cause of the many false positive results. A prime consideration in method development is the accurate determination of low concentrations of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD). Using the EPA-1613 standard calibration set (0.5 - 200 pg/µl), a calibration curve was constructed for the seventeen congeners. The 2,3,7,8-TCDD calibration curve obtained was linear (R2 = 0.9996); and an average response factor of 1.06 was obtained. The quantitation capability was further investigated using the low-level standard (0.5 pg/µl) to calculate a signal/noise (S/N) ratio for the ion of mass to charge ratio (m/z) 322 for 2,3,7,8-TCDD. The resulting S/N of 20 is well above that set by EPA Method 1613 (>10). To confirm the limit of detection (LOD) in soils, two 10 g confirmed blank soil samples were spiked with native dioxins at concentrations of 0.5 pg/µl and 200 pg/µl for 2,3,7,8-TCDD. After the addition of labeled material, extraction, clean-up (GPC) and concentration, it was possible to calculate LODs for 2,3,7,8-TCDD of 322 fg and 353 fg respectively. These calculations were made by determining the S/N for the ion of m/z 322 and extrapolating linearly to an S/N of 3:1. Results were consistent with the LOD determined from the lowest calibration standard, thus providing assurance that the method has the sensitivity necessary for low-level dioxin determination.
We now have a combined method for the analysis of dioxins and furans in soil and sediment samples. It uses the H4IIE-luc bioassay as a preliminary screening tool, reducing the need for unnecessary instrumental analysis and driving down the cost per sample. Analysis of positive samples is conducted using GC×GC-TOFMS, which provides dioxin sensitivity at the required levels. In addition, since this is not a target compound technique it is capable of providing information on multiple compound classes present in the sample in one run, giving a comprehensive picture of sample toxic potential. A lack of access to certain analytical resources means that South Africa is not always able to quickly assess and respond to POP emergencies, which could negatively affect human and environmental health, as well as trade and industry. South Africa is not alone. We hope we have emphasized a real need to develop local analytical capability that employs regionally relevant methods to generate internationally acceptable results. Our end result is a method that is time-efficient, financially viable, and suitable for use in developing economies where instrumental availability, skills and finances are often limited.
Jayne de Vos is Director of Chemistry at NMISA, Pretoria, South Africa (bjdevos@nmisa.org) and Peter Gorst-Allman is Director, Applications at LECO Africa, Kempton Park, South Africa (peter@lecoafrica.co.za).
References
- POPs, PAHs, and elemental levels in sediment, fish and wild bird eggs in the Orange-Senqu River basin. Report 002/2013, prepared by H. Bouwman, R. Pieters, B. Genthe and J. Chamier for ORASECOM, Pretoria. R. Dixon et al., Crocodile Deaths in the Kruger National Park (http://tas.txp.to/901-Kruger) J. de Vos et al., “Comprehensive two-dimensional gas chromatography time of flight mass spectrometry (GCxGC-TOFMS) for environmental investigations in developing countries”, Chemosphere, 82, 1230 (2011 J. de Vos, P. Gorst-Allman, and E. Rohwer, “Establishing an alternative method for the quantitative analysis of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for developing countries”, Journal of Chromatography A, 1218, 3282 (2011). E. Hoh, K. Mastovska, and S. Lehotay, “Optimization of separation and detection conditions for comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry analysis of polychlorinated dibenzo-p-dioxins and dibenzofurans”, Journal of Chromatography A, 1145, 210 (2007).
