Chris Wright, Head of Analytical Science at British American Tobacco (BAT) Group R&D (UK), has worked in analytical chemistry for over 30 years. Starting as a government scientist measuring dioxins in food and human tissues, he later spent 10 years at Unilever, helping to ensure the safety of the company’s food and cosmetic lines. Looking for a different analytical challenge, he joined BAT in 2008, despite raised eyebrows from some of his colleagues. “There were people who I had worked with for years who reacted angrily to the move. We all know that the tobacco industry has a checkered history when it comes to ethics and transparency, and I wasn’t blind to that. But I saw changes happening in the industry, not least a move away from conventional cigarettes and towards less harmful alternatives,” says Wright. His misgivings were lessened when he met the R&D team at BAT and found them very frank about the dangers of tobacco smoking. “I heard countless statistics about the impact of smoking on health and mortality – there was no shying away from the inherent toxicity of tobacco,” he says.
When Wright joined BAT, he says the analytical testing in the industry was still relatively low-tech, lagging behind the prevailing standards in food and environmental analysis. So he spent three years with a small team working to improve the robustness of tobacco and cigarette smoke analysis, before being presented with a new challenge: how to characterize complex aerosols from novel ENDS. “I had always been interested in non-targeted screening of foods, including working with the International Life Sciences Institute on the application of the ‘Threshold of Toxicological Concern’ concept to food chemical residues. Suddenly, I had an opportunity to do something similar in a new field, with a potentially significant impact on public health,” he says.
Attack of the vapors
Now, Wright guides R&D on technical standards, selection of analytical techniques, strategic direction for analytical science and investment in analytical technologies. He also works closely with the company’s toxicologists to ensure that the department provides robust data for product assessments. Analyzing aerosols from e-cigarettes or heated tobacco products poses significant challenges for both chemical and biological analyses. The analytical team seeks to answer questions from ‘How does this work?’ through to ‘What substances are formed when…?’ to ‘How safe is this?’. But, says Wright, “The biggest question facing the team right now is ‘How many substances can we detect and identify simultaneously in aerosols?’”

E-cigarette vapor is typically analyzed by GC but Wright’s team are now introducing HPLC-based techniques. “When we started out, the assumption was that all e-cigarette aerosols were vapor, which would be most easily analyzed by GC. Subsequently, we found that 90–95 percent of an e-cigarette aerosol can be collected on a glass filter pad milliseconds after it is formed – in other words, it condenses very quickly.” A few years ago the team acquired two Bruker maXis high-resolution LC-TOF instruments, which are proving a welcome addition to confirm results obtained by GC. “We are also exploring real-time analytical tools, such as SIFT-MS (Syft), which allow instant monitoring of substances in aerosols and potentially rapid or at-line chemical characterization,” says Wright. Real-time analysis would benefit the product development team in particular, giving them immediate information to make go/no go decisions during early-stage design. On the biological side, some of the in vitro assays used in toxicology have proved difficult to apply to vaping. “The humectants used in e-cigarettes (such as propylene glycol) absorb water very well, so when added to an in vitro system, they cause dehydration and shock – obscuring some of the toxicology,” he explains. “That’s one reason why much of research so far has focused on chemical rather than biological screening, but I hope to see more sophisticated biological endpoint testing being applied to e-cigarettes soon.”
Another dimension
An ongoing collaboration with Jef Focant at the University of Liege, Belgium has brought multidimensional GC analysis into the company’s analytical armory. “I first met Jef over 20 years ago, when we were both working on dioxins. Jef went on to specialize in the emerging area of GC×GC – the only technique we thought would have the chromatographic peak capacity to separate aerosols as complex as cigarette smoke or e-cigarette vapor,” says Wright. Initially, the project was about feasibility, and concentrated on conventional cigarettes. “There had been a few publications from other tobacco companies, showing some great separations but lots of problems with overloading and dynamic range,” says Wright. It’s a tall order to catalog every compound in cigarette smoke, not least because of the sheer volume of data generated. So the team focused on data crunching tools that detect differences, rather than analyzing every component. “We made small changes – for example, changing the adsorbents in the cigarette filter – and looked for changes in the smoke chemical profile, which allowed us look at cause and effect on a chemical level, in bite-sized chunks,” says Wright. “Our collaboration has generated some very insightful and visually striking data to distinguish even small differences in very complex aerosol samples” (1)(2)(3)(4).
Big in Japan
BAT’s e-cigarette platform is relatively stable in terms of products and acquisitions, so these days analytical activities focus on managing future commercial and regulatory pressures. Currently, the team spends most of its time on the company’s tobacco heating (also known as heat-not-burn) products: Glo. “These products are proving to be a huge commercial success, and it’s important that we ensure that they are as safe as they possibly can be,” says Wright. The products have proved especially popular in markets like Japan, where nicotine-containing e-liquids are restricted, and where cultural values of discretion and consideration for others make ENDS appealing. “In a conventional cigarette you get combustion and a lot of pyrolysis, whereas heated products induce something akin to torrefaction of the material, releasing only the more volatile compounds as an aerosol,” says Wright. Those volatile components include nicotine and various flavor compounds, but largely exclude the combustion products that are major contributors to the toxicity of conventional cigarettes.The vapers of the future
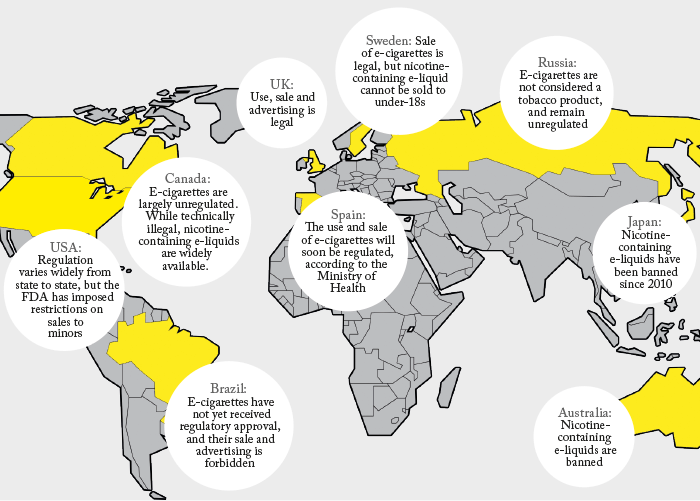
Currently, although EU law mandates data reporting, there is no common standard for ENDS and Wright believes there may be products on the market that carry a risk of unexpected and avoidable chemical hazards. Though BAT state that they include only compounds that have a known toxicity profile in their e-liquids, that isn’t necessarily the norm in what is a largely unregulated business in much of the world. “I would like to see ENDS become more formally regulated, to provide consistency in technical standards, harmonized methods for sample actuation, aerosol generation and physical/chemical testing,” Wright says. “This would provide direct assurance to consumers and regulators that the products that will replace cigarettes have been rigorously designed and that their long-term health impacts have been fully evaluated.” To date, the tobacco industry has focused on comparative risk reduction, but Wright believes more can be done to characterize the residual risk. “Just because a substance appears in lower levels in vapor than in smoke doesn’t mean it isn’t a health risk. Ideally, we need to set threshold levels for each compound, so that we can concentrate our efforts on those compounds that remain above threshold. To do that, we need sensitive analytical instruments and powerful data analysis software.”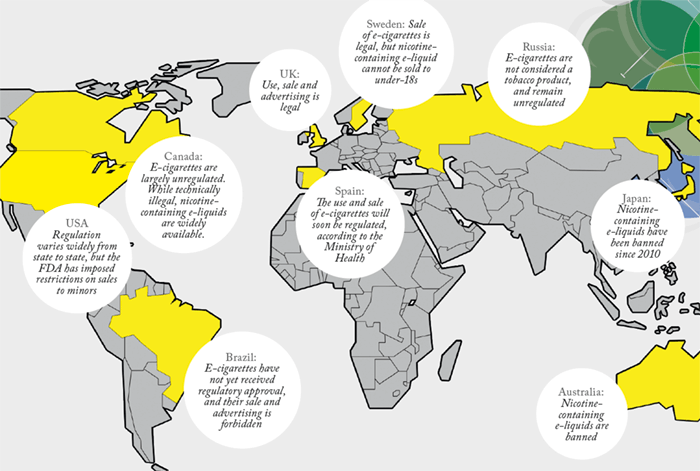
This article has three parts: Analyzing Uncertainty, featuring Hugo Destaillats and Mohamad Sleiman Industry Insights, featuring Chris Wright The Human Element, featuring Lion Shahab
References
- B Savareear et al., “Thermal desorption comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry for vapour phase mainstream tobacco smoke analysis”, J Chromatogr A, 1525, 126–137 (2017). B Savareear et al., “Headspace solid-phase microextraction coupled to comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the analysis of aerosol from tobacco heating product”, J Chromatogr A, 1520, 135–142 (2017). M Brokl et al., “Multivariate analysis of mainstream tobacco smoke particulate phase by headspace solid-phase micro extraction coupled with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry”, J Chromatogr A, 1370, 216–229 (2014). M Brokl et al., “Analysis of mainstream tobacco smoke particulate phase using comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry”, J Sep Sci, 36, 1037–1044 (2013).




