Introduction
Aggregation of proteins has been an intense area of study largely due to the growth of the biotechnology industry. As protein therapeutics are developed, aggregation must be monitored and reduced as it can potentially cause immunogenicity1 as well as a possible decrease in activity.2,3 One of the ways aggregation is controlled is through the use of osmolytes as excipients.4 Many excipients have been shown to induce stability through preferential exclusion. A particular subclass of these molecules includes polyols and carbohydrates.5,6 Polyols are typically added to protein formulations in order to increase tonicity and improve stability. The addition of polyol osmolytes has been shown to prevent aggregation, retain activity, inhibit chemical degradation, enhance the refolding of proteins,7 and increase the thermal stability of proteins.4-6,8-11 It has been shown that the more hydroxyl groups on the carbon chain of the polyol, the greater the thermodynamic stability that can be induced. This has been attributed to an entropic effect where the polyol is excluded from the surface of the protein. The polyol has a greater impact on the water-peptide interactions than it does on the interactions with the nonpolar groups that are exposed when the protein is denatured.12To date, no systematic study has been performed using multiple proteins to address whether different stereoisomers of a polyol would induce more or less thermal stability on a protein. The goal of the study was to understand how the size of a polyol and orientation of the hydroxyl group influence the amount of thermal stability that could be induced upon seven monoclonal antibodies of different isotypes. In order to monitor thermal stability, differential scanning calorimetry (DSC) has been employed. DSC has been used to monitor the unfolding of proteins due to heat denaturation in order to study the unfolding of the domains of an antibody,13 monitor thermal reversibility,14-16 and screen formulations for excipients.17,18
Experimental procedures
Materials Seven monoclonal antibodies of varying isotypes were obtained. Two antibodies were κIgG1 (K1A and K1B); two were λIgG1 (L1A and L1B); two were κIgG2 (K2A and K2B); and one antibody was an κIgG4 (K4). Histidine, glycerol, erythritol, ribitol, xylitol, mannitol and sorbitol were obtained. For thermal denaturations, a high throughput DSC equipped with an auto-sampler was used. Sample preparation Each antibody was dialyzed against RODI water. Stock solutions of histidine, glycerol, erythritol, ribitol, xylitol, mannitol and sorbitol were prepared. A liquid handling robot was used to prepare antibody solutions of 1.25 mg/mL in 10 mM histidine at pH 6.0. A titration was performed of 0-350 mM polyol concentration. Buffer blanks were also prepared. Thermal denaturations Each sample was subjected to heat denaturation by scanning the samples and their buffer controls from a temperature range of 30 to 95°C at 60°C/hr. The thermograms were baseline-subtracted and deconvoluted using the software provided by manufacturer to obtain the thermal midpoints (Tm) of each transition. Each antibody was fit to three or four transitions depending on how well the unfolding of the Fab and Fc domains were resolved from each other. Figure 1 depicts the three unfolding transitions of L1B where the CH2 domain and one of the Fab domains are not resolved.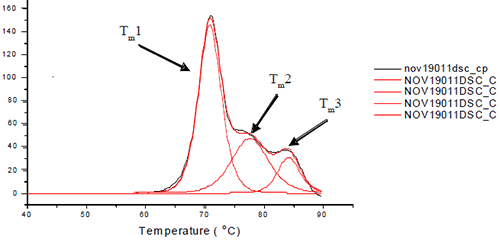
Figure 1. Representative thermogram for L1B. For L1B, Tm1 is a combination of the unfolding of the CH2 and one of the Fc domains. Tm2 is the unfolding of the CH3 domain, and Tm3 is the unfolding of the other Fc domain. Note that the order in which the domains unfold may change for different antibody isotypes. >> Download the full Application Note as PDF
Malvern provides the materials and biophysical characterization technology and expertise that enables scientists and engineers to investigate, understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. www.malvern.com Material relationships http://www.malvern.com/en/ portal@malvern.com





