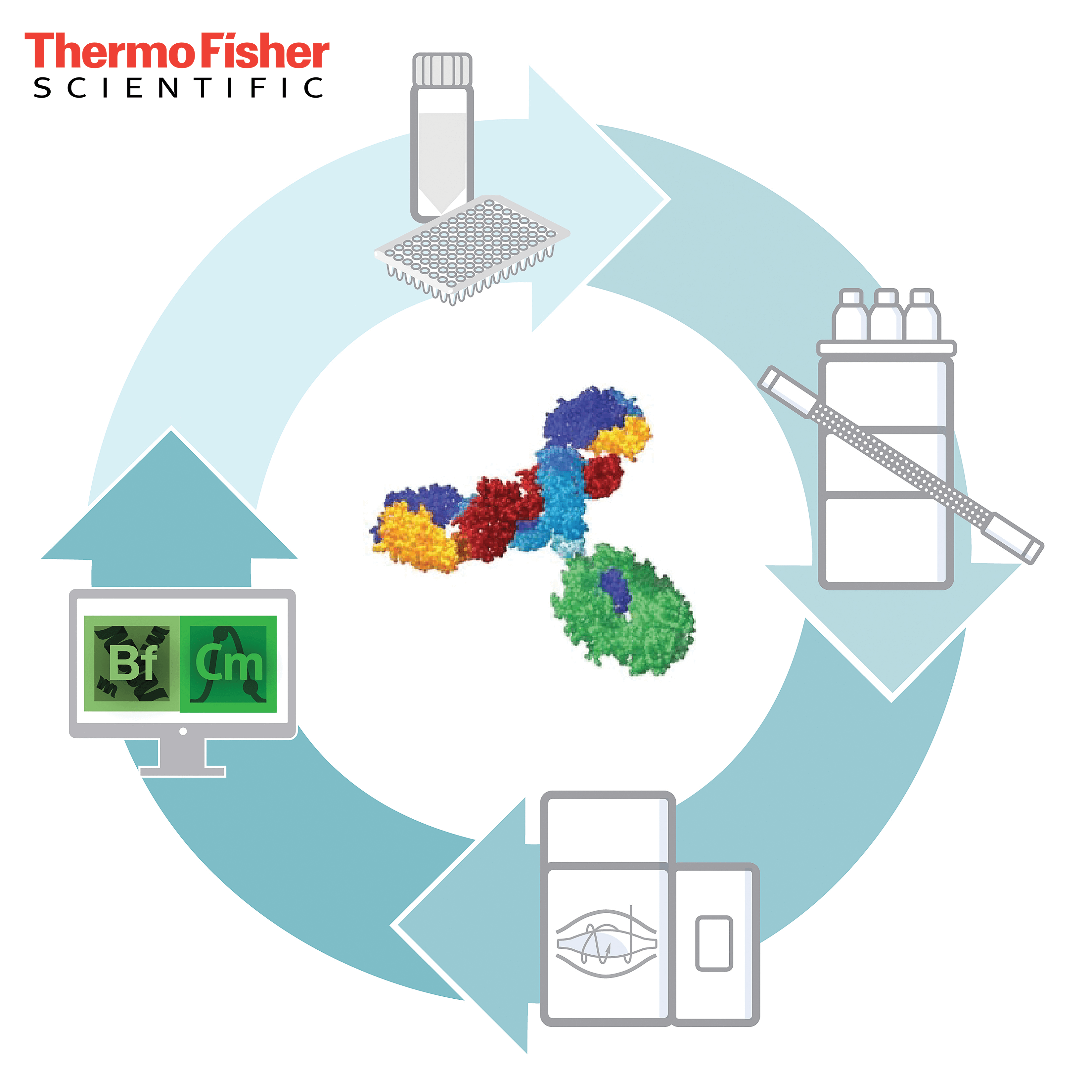
The Thermo Scientific HR Multi-Attribute Method (MAM) is a powerful high resolution accurate mass (HRAM)-based workflow that enables comprehensive characterization and monitoring of quality attributes from research to quality control. The HR MAM consists of industry-leading hardware and compliance-ready software as well as a system suitability test specifically developed for the workflow. Built within a safe, compliant environment, the HR MAM enables characterization of biologics with ease and allows potential critical quality attributes to be monitored throughout every stage of the drug development process, while also providing purity testing with the feature of new peak detection.