Over the last few years, several coupling techniques for mass spectrometry have emerged to broaden the scope of TLC-MS analysis beyond elution-based sampling. Though some ambient MS techniques were not developed specifically for TLC, they offer additional flexibility and a number of advantages. However, with choice comes the potential for confusion! We hope to shed light on what is available, comment on the level of “commercialization” or development, and also indicate the most appropriate choice, depending on your objective or application.
Over the last few years, several coupling techniques for mass spectrometry have emerged to broaden the scope of TLC-MS analysis beyond elution-based sampling. Though some ambient MS techniques were not developed specifically for TLC, they offer additional flexibility and a number of advantages. However, with choice comes the potential for confusion! We hope to shed light on what is available, comment on the level of “commercialization” or development, and also indicate the most appropriate choice, depending on your objective or application.
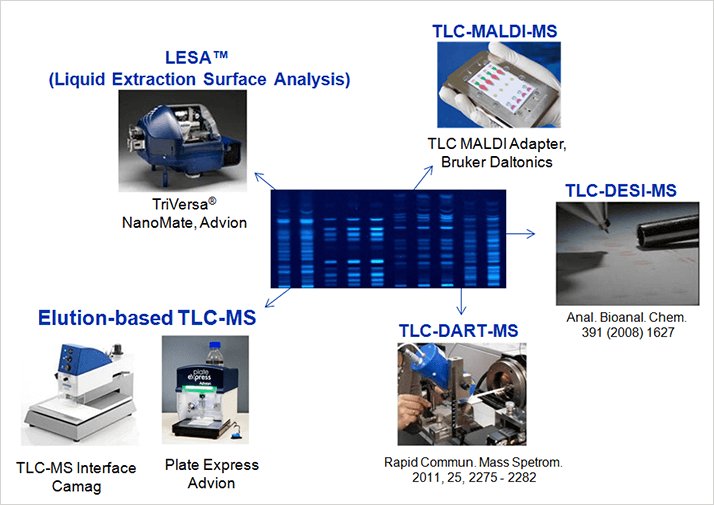
TLC-MALDI-MS
Matrix-assisted laser desorption/ionization (MALDI)-MS is well respected and has found widespread use in a number of areas, such as tissue imaging and proteomics. MALDI’s ability to scan an area of interest make it a natural partner for TLC; to that end, Bruker Daltonics introduced an adapter that allows you to directly insert your TLC plate into a MALDI instrument. The fully automated measurement process allows an entire plate to be scanned and produces a visual representation of separations. Indeed, the data evaluation software enables so called MALDI chromatograms that plot molecular mass against TLC position, producing a two-dimensional view; analytes that overlap on the TLC plate are separated by mass and shown in a different color. From an application perspective, TLC-MALDI-MS is strongly suited to proteins, peptides and lipids, especially when analyzing less complex mixtures. And though it is unlikely to overthrow gel electrophoresis methods, in certain applications it is a compelling additional analytical tool. Notably, MALDI matrix must be applied ahead of analysis, and only aluminum backed plates may be used because of the need for plate conductivity.TLC-DART-MS and TLC-DESI-MS
Like MALDI, direct analysis in real time (DART) and desorption electrospray ionization (DESI) are general surface analysis techniques and therefore also lend themselves to TLC analysis. The mode of operation at the surface of the plate is somewhat similar, but in DART-MS a gas stream is focused on the TLC plate; in DESI, the stream hitting the surface is a solvent mixture. Also like MALDI, both techniques are able to scan the entire plate, which offers distinct advantages. Gertrud Morlock has published a number of papers on TLC-DART-MS (1). Morlock is a real advocate of TLC-MS in general, and you can read her opinions in the first article of this series online: tas.txp.to/0715/missinglinkWe haven’t done a great deal of work with TLC-DART-MS or TLC-DESI-MS (2) yet, but that’s not to say they are uninteresting techniques – to the contrary. However, while the sources are commercially available, right now there are simply no complete systems available to enable straight-forward coupling, which is why academics like Morlock are leading the way. Our experience of these techniques comes from collaboration with such groups and we have been impressed with the results. Certainly, these two techniques are seriously worth following.
Liquid Extraction Surface Analysis
LESA™ technology was originally developed to investigate tissue slices, but it can analyze almost any surface with its nano-robotic ESI source – and that includes TLC plates. The TriVersa® NanoMate (Advion) automatically works its way across the plate, taking a fresh pipette tip to analyze each “zone,” which practically eliminates carry over. In actual fact, the robot is capable of multiple modes of operation, but for LESA the robot picks up a pipette tip, draws extraction solvent from a reservoir, moves to the zone of interest, allows a small droplet of solvent to mix with the sample spot for a preset time, and draws up the mixture before nanospray injection into any high-end MS system. LESA is an elegant solution that offers high sensitivity. And the high level of automation is welcome for high-throughput studies. The system does however require hydrophobic plates to operate effectively, so reverse-phase (RP) modified TLC plates are needed. Fortunately, RP plates are suitable for most separations. In fact, we have run a number of applications using LESA and in 80-90 percent of cases, it works very well. There is, however, a sort of paradox: the nanospray infusion of the TriVersa NanoMate works best coupled with high-end mass spectrometers – but in general, laboratories that have such instruments do not typically perform TLC analysis. That said, because the technique is so promising, we are keeping a keen eye on further developments. Certainly, as the TLC-MS user base expands, we can imagine a number of advanced labs adopting this approach.Elution-based TLC-MS
Two commercial systems are available for elution-based TLC-MS, both of which were developed specifically for the task. Morlock introduced the concept (and the background) to CAMAG’s Interface in the article noted earlier, and very recently Advion released its Plate Express. Plate Express operates on a similar principal to CAMAG’s Interface – both utilize a head that seals to the plate with a cutting edge. A capillary channel introduces solvent to the spot within the seal, and another channel extracts the analytes and solvent for introduction into a mass spectrometer. Perhaps the strongest feature of elution-based coupling is its complete independence from the mass spectrometer – any MS system can be used, in principal. Plus, offline extraction into vials is possible, if additional analysis (with NMR, for example) is needed. In online TLC-MS mode, the interface sits between the HPLC pump and the MS system, and is pretty much “plug and play”. It’s fair to say that such systems offer the lowest entry barriers into TLC-MS, and can be used with almost any kind of plate. However, unlike MALDI, DART and DESI, these elution-based approaches don’t allow scanning of the whole plate, instead taking measurements from each sample zone within the head’s cutting edge.TLC’s renaissance
Hopefully, a clear picture is emerging: TLC-MS is a growing and evolving field. With a number of very promising – but quite different – solutions on the market, it’s clear that there is great value in combining the power of TLC and MS in a number of different application areas. Indeed, the number of publications focusing on TLC-MS is steadily increasing, and as the technique becomes more affordable (the price – and size – of MS systems is coming down) we expect the upward trend to continue. TLC is already a very low-cost analysis method – add low cost mass spectrometry, and it becomes an compelling orthogonal tool that is hard to ignore. A strong driver for more rapid development will simply be increased uptake of TLC-MS – although there is growing interest, many people are still not aware of the potential of the technique, which impedes progress. But things are changing. We recently held a (national) TLC-MS user meeting in Darmstadt, Germany, and although it was relatively small, it still drew a similar number of delegates – more than 100 – to the last HPTLC symposium in Lyon. Why? Because it attracted both TLC and MS users, particularly those who are interested in high-quality quantitative screens or orthogonal analytical methods. Likewise, at mass spectrometry conferences it’s very interesting to follow new developments in ambient ionization for MS – in principal, many of these can most likely be applied to TLC in innovative ways. Are we at the beginning of a virtuous cycle of dedicated instrument development for TLC-MS? We think so and believe a thriving community will quickly emerge. Find out “what you’ve been missing” with Michael Schulz’s comprehensive webinar tour of TLC-MS, moderated by Rich Whitworth (Editor): tas.txp.to/0715/TLCwebinarBroadly speaking, TLC-MS offers three key benefits: the ability to handle matrix rich samples, orthogonality to HPLC, and parallelization. Here, we share some interesting research from the past few years that highlights the potential of applying TLC-MS in your lab. TLC-DART-MS “Combined multivariate data analysis of HPTLC fingerprints and direct analysis in real time mass spectra for profiling of natural products like propolis” G. E. Morlock et al., J. Chromatogr. A, 1328, 104–112 (2014). tas.txp.to/0715/TLC1 Elution-based TLC-MS “Characterization of saponins in peas (Pisum sativum L.) by HPTLC coupled to MS and a hemolysis assay” V. Reim and S. Rohn, Food Res.Int. (2014). tas.txp.to/0715/TLC2 Elution-based TLC for sample preparation “Planar solid phase extraction clean-up for pesticide residue analysis in tea by liquid chromatography–mass spectrometry” C. Oellig and W. Schwack, J. Chromatogr. A, 1260, 42-53 (2012). tas.txp.to/0715/TLC3 TLC-MALDI-MS “Stationary phase thickness determines the quality of TLC-MALDI-MS of lipids” H. Griesinger et al., Anal. Biochem., 451, 45–47 (2014). tas.txp.to/0715/TLC4 TLC-MS still evolving: “direct spray” MS “Identification and semi-quantitative determination of anti-oxidants in lubricants employing TLC-spray MS” G. Kreisberger, J. Chromatogr. A, 1383, 169–174 (2015).
References
- E. S. Chernetsova, A. I. Revelsky, and G. E. Morlock, “Some New Features of Direct Analysis in Real Time Mass Spectrometry Utilizing the Desorption at an Angle Option”, Rapid Commun. Mass Spectrom. 25: 2275–2282 (2011). DOI: 10.1002/rcm.5112 S. P. Pasilis et al., “Using HPTLC/DESI-MS for Peptide Identification in 1D Separations of Tryptic Protein Digests”, Anal. Bioanal. Chem. 391(1), 317-24 (2008). DOI: 10.1007/s00216-008-1874-6




