Introduction
Distillers grain (DG), a major coproduct of dry-grind ethanol processing, quadrupled in production during 2004–2005. It is currently the second largest category of processed feed in the U.S., with an estimated 35 million metric tons (tonnes) produced in 2011. Nearly 25% of U.S. DGs are exported and the primary markets are China, Mexico, and Canada. DG is a valuable product to the livestock industry because it is a rich source of protein, fat, minerals, and vitamins, thus making it an excellent feed supplement for livestock and poultry.1–4 However, bacterial contamination from lactic acid-producing bacteria—such as Lactibacillus, Lueconostoc, and Weissella—is a concern for ethanol production facilities because bacteria compete with yeast for sugar and micronutrients. Antibiotics such as virginiamycin, penicillin, and erythromycin are commonly used during fermentation to inhibit bacterial growth.5

Although the U.S. Food and Drug Administration (FDA) is responsible for regulating all drugs and ingredients used for animal feed production, there is currently no active enforcement for antimicrobials used in DG products. The FDA has raised concern over foodproducing animals consuming DGs with antibiotic residues, and how this may lead to increased antibiotic resistance in humans and animals. Therefore, to assess the amount of antibiotics in DGs and to meet possible future regulatory requirements, analytical methods are needed to determine residual antibiotics in DGs.

This study discusses the determination of penicillin G, erythromycin, and virginiamycin S1 and M1 in dried distillers grains with solubles (DDGS). These compounds (Figure 1) are the four major antibiotics used in ethanol production and belong to different antibiotic classes that include β-lactams, macrolides, and streptogramins. Their different physical and chemical properties increase the challenge to extract, separate, and detect these compounds in a single analysis.6–8 For example, penicillin is hydrophilic in nature and therefore is easily extracted from DDGS with water. However, erythromycin and virginiamycin M1 and S1 are insoluble or only slightly soluble in water and therefore require an organic solvent for extraction.
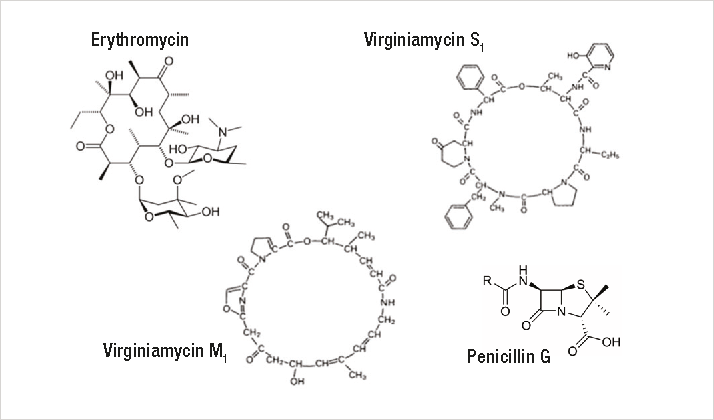 Figure 1. Structures of antibiotics used in the ethanol production process.
Figure 1. Structures of antibiotics used in the ethanol production process.The challenges are further magnified because UV detection lacks the sensitivity to detect some of these antibiotics because either they do not have a chromophore or have only a weak chromophore (e.g., virginiamycin and erythromycin). One approach to overcome this problem is to use a Thermo Scientific™ Dionex™ Corona™ ultra RS™ Charged Aerosol Detector. This mass-sensitive detector provides good sensitivity of nonvolatile and some semivolatile analytes that lack a strong chromophore. After sample preparation, the four antibiotics are separated from the remaining components of the DDGS sample using a Thermo Scientific™ Acclaim™ 300 C18 column and then detected by charged aerosol detection. This method allows accurate determination of these antibiotics in DDGS.





