Summary
This application note describes a Solid Phase Extraction (SPE) protocol for the extraction of a range of mycotoxins from wheat flour, wheat, maize and barley using ISOLUTE® Myco with LC-MS/MS.
Introduction
Mycotoxins are toxic metabolites produced by fungal molds on food crops. Regulation and legislation for testing of mycotoxin contamination has established which mycotoxins are preva- lent on a wide variety of food crops. This application note describes an SPE protocol appropriate for LC-MS/MS analysis of a range of mycotoxins found on grain food crops.
 Figure 1. Structures of Aflatoxin B1 and Zearalenone
Figure 1. Structures of Aflatoxin B1 and ZearalenoneThe method described in this application note achieves high recoveries of all relevant mycotoxins from a range of different grain matrices with %RSDs and LOQs that all meet the require- ments set in European regulations for measurement of these analytes in grains. ISOLUTE Myco solid phase extraction columns provide robust, reliable sample preparation for multiple mycotoxin classes from a wide range of foodstuffs. Using a single, easy to use sample preparation product, along with optimized matrix specific application notes, scientists can prepare diverse food/crop samples for analysis by LCMSMS.
Analytes
Aflatoxin B1, aflatoxin B2, aflatoxin G1, aflatoxin G2, ergocryptine, ergocornine, ochratoxin A, fumonisin B1, zearalenone, T-2 mycotoxin, HT-2 mycotoxin
Sample Preparation Procedure
Column configuration Sample pre-treatment:
ISOLUTE Myco 60 mg/3 mL column (Tabless) Part Number 150-0006-BG Sample pre-treatment:
- Sample processing: Grind the sample (wheat, maize, barley, 50 g). Store ground sample in a sealed container at room temperature until required.
- Extraction: Mix the ground whole grain (or flour) sample (5 g) with 50% acetonitrile (aq) (20 mL) and place on a shaking table for 30 minutes. Transfer the extract to a 50 mL centrifuge tube and centrifuge at 3000 g for 10 minutes.
- Dilution: Take the supernatant (8 mL), transfer to a new 50 mL centrifuge tube and dilute with water (32 mL). Centrifuge diluted extract at 3000 g for a further 10 minutes.
Solid Phase Extraction
Use flow rates of 1 mL min-1 throughout Condition:
Condition the column with acetonitrile (2 mL) Equilibration:
Equilibrate column with water (2 mL) Sample loading:
Load pre-treated sample (3 mL) onto the column at a maximum flow rate of 1 mL min-1 (gravity load is recommended) Interference wash 1:
Wash the column with water (3 mL) Interference wash 2:
Wash the column with 10% acetonitrile (3 mL) Drying:
Dry the column for 30 seconds at maximum vacuum Elution 1:
Elute with 0.1% formic acid in acetonitrile (2 mL) Elution 2:
Elute with methanol (2 mL) Post elution:
The combined eluate is dried in a stream of air or nitrogen using a SPE Dry (35 °C, 20 to 40 L min-1) or TurboVap LV (15 bar at 35 °C for 40 min). Reconstitute in 0.1 % acetic acid in 20% acetonitrile : methanol (1 mL, 1:1, v/v). Syringe-filter using a 0.2 μm PTFE membrane prior to analysis.
HPLC Conditions
Instrument:
Shimadzu Nexera UHPLC (Shimadzu Europe Gmbh) Column:
Kinetex XB-C18 50 x 2.1 mm 2.6 μm dp (Phenomenex, Macclesfield UK) Mobile Phase:
A: 1 mM ammonium acetate, 0.5% acetic acid
B: 1 mM ammonium acetate, 0.5% acetic acid in 95% methanol (aq) Flow rate:
0.45 mL min-1 Injection:
20 μL Gradient:
Initial 20 % B, hold 1.0 min
linear ramp to 73 % B in 6 min
linear ramp to 100 % B in 0.2 min, hold 2.3 min
linear ramp to initial conditions in 0.2 min
hold 2.3 min, total run time 10.0 min Column temperature:
40 °C Sample temperature:
15 °C
MS Conditions
Ions were selected in order to achieve maximum sensitivity, and the MS was operated in dual polarity (+ve/-ve switching) mode, using multiple reaction monitoring. Instrument: AB Sciex Triple Quad 5500 (Warrington, UK) Source: Turbo-V ESI Desolvation tempurature: 500 °C Curtain gas: 30 psi Spray voltage: +5.0 kV / -4.5 kV Gas 1: 60 psi Gas 2: 60 psi Collision gas: 7 psi
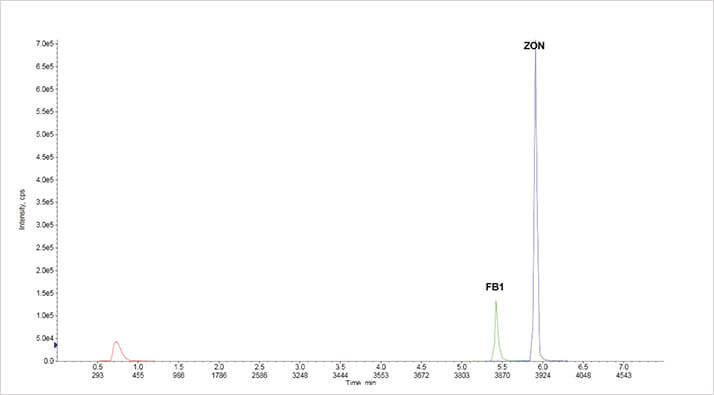 Figure 2: Extracted ion chromatograms in negative ion mode using ISOLUTE Myco protocol at 50 μg kg-1 from wheat
Figure 2: Extracted ion chromatograms in negative ion mode using ISOLUTE Myco protocol at 50 μg kg-1 from wheat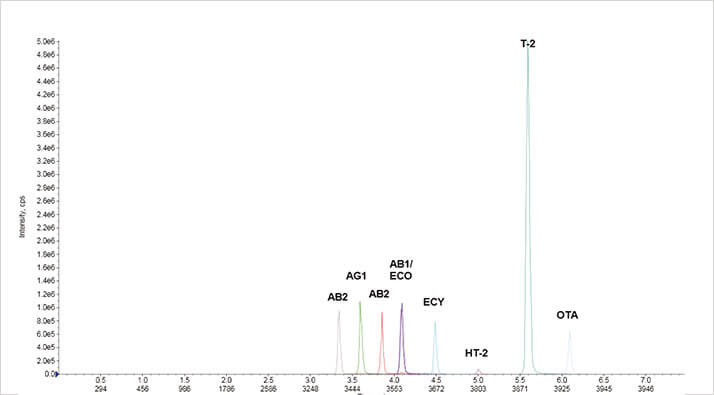 Figure 3. Extracted ion chromatograms in positive ion mode using ISOLUTE Myco protocol at 5 μg kg-1 (aflatoxins and ochratoxin A) and 50 μg μg kg-1 (others) from wheat grain
Figure 3. Extracted ion chromatograms in positive ion mode using ISOLUTE Myco protocol at 5 μg kg-1 (aflatoxins and ochratoxin A) and 50 μg μg kg-1 (others) from wheat grainValidation Criteria
Method linearity was determined using matrix-matched calibration standards in six replicates over a minimum of five levels (the majority were determined with seven levels); the ranges are shown below.LOQ was determined from the lowest matrix-matched standard meeting EU repeatability and recovery criteria. Where no criteria were specified the LOQ criteria were estimated by correlation to similar analytes. Repeatability (%RSDr) was determined from single acquisitions of 5 SPE replicates of a single sample extraction. The RSDs generated gave close agreement when a single sample was extracted and processed using ISOLUTE Myco from three separate sorbent batches. Recovery was determined as a % of ISOLUTE Myco extract spike before sample prep to spike after at the EU MRL.
Results
The extracted ion chromatograms in figures 2 and 3 demonstrate chromatography at 5 μg kg-1 (aflatoxins and ochratoxin A) and 50 μg kg-1 for all other analytes from a spiked extraction of 10 g ground wheat. Good linearity was achieved for all analytes in all the different matrices as demonstrated in the example charts shown in figures 4 and 5.
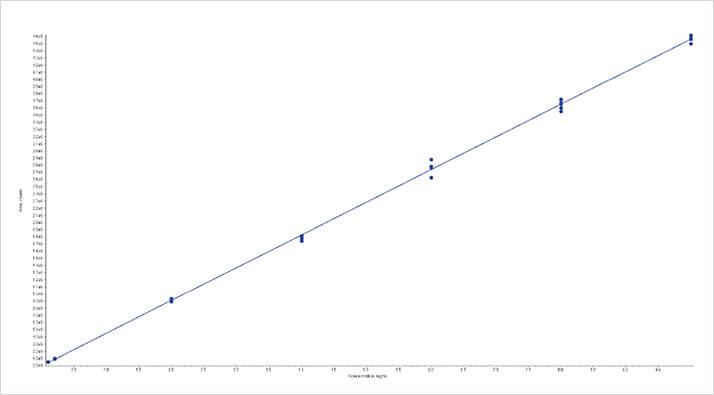 Figure 4: Calibration curve for aflatoxin B1 from ground wheat using the ISOLUTE Myco protocol from 0.1 – 10 ngmL-1
Figure 4: Calibration curve for aflatoxin B1 from ground wheat using the ISOLUTE Myco protocol from 0.1 – 10 ngmL-1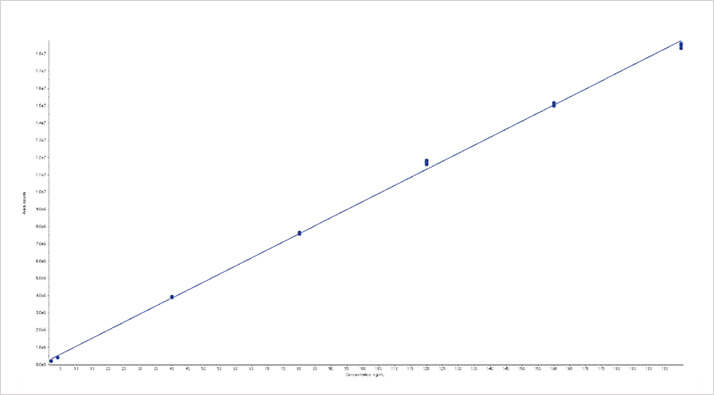 Figure 5. Calibration curve for T2 from ground wheat using the ISOLUTE® Myco protocol from 5 – 200 ngmL-1
Figure 5. Calibration curve for T2 from ground wheat using the ISOLUTE® Myco protocol from 5 – 200 ngmL-1All analytes extracted using the ISOLUTE Myco protocol achieved the limits of quantities and recovery required by the current European standards for mycotoxin analysis as shown in tables 4, 5 and 6.
Ordering Information
Download the PDF of this App Note here.
For the latest application notes and more information about ISOLUTE® Myco, please visit www.biotage.com/isolutemyco





