Choosing a Carrier Gas for GC
The aim within the laboratory should be to achieve the best separation in the shortest time period. The most commonly used gases as carrier gas for GC are nitrogen, hydrogen and helium.The differences between the gases are evident when comparing their van Deemter curves. This is illustrated in the Van Deemter Equation (Figure 1).

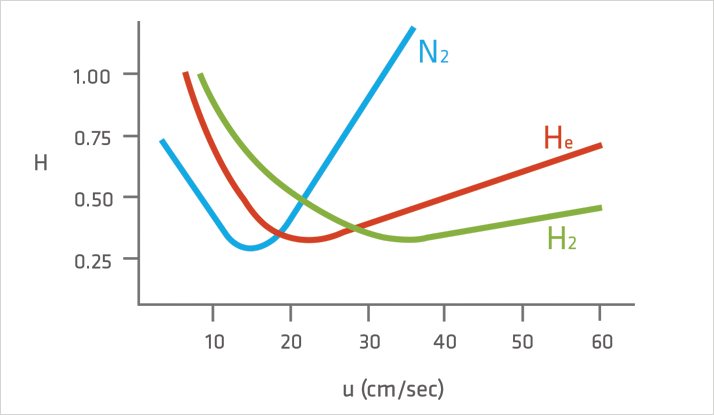 Figure 1: The Van Deemter Equation
Figure 1: The Van Deemter EquationAlthough it displays the lowest minimum plate height compared with that of helium or hydrogen, nitrogen has a much narrower velocity range and a steeper van Deemter curve, so at higher flow rates, solute efficiency drops off dramatically (Figure 1). Analysis times with helium are about 1/2 the value when using nitrogen and there is only a very small sacrifice in efficiency. The helium Van Deemter curve is much flatter than the nitrogen curve, thus changes in the average linear velocity do not decrease efficiency by a large amount. Hydrogen is the fastest carrier gas (uopt), with an optimum linear velocity of 40cm/sec, and exhibits the flattest Van Deemter profile. Hydrogen’s high uopt (optimal linear velocity) results in the shortest analysis times. Also, the wide range over which high efficiency is obtained makes hydrogen the best carrier gas for samples containing compounds that elute over a wide temperature range.Although Nitrogen, Helium and Hydrogen can all be considered suitable carrier gases for use in GC, historically helium has been the most widely used due to the safety concerns associated with hydrogen and also the fact that nitrogen is much less efficient!
Why Change from Helium
The emerging helium shortage means that people have no choice but to look at other alternatives. Are we running out of Helium completely? No, not quite yet but a shortage of helium and increased demand within the medical, scientific and industrial fields is leading to this rare commodity rising in price. Many leading GC Manufacturers understand the impact this may have in the laboratory and have started to actively recommend switching your carrier gas from Helium to Hydrogen.
Using Hydrogen as a Carrier Gas for GC
There are major benefits of using Hydrogen as your carrier gas:
- Increased speed: increasing the linear flow rate allows for shorter run times, thereby increasing the throughput of your laboratory.
- Lower temperature separations: at the faster elution times, it may not be necessary to increase the column temperature run rate. It may even be possible to lower the maximum temperature needed for the analysis.
- Longer column life: lower temperatures lead to less column bleed, which in turn can mean a longer column life. In addition, hydrogen is a reducing gas and can remove potential acidic sites off the column, further increasing column life!
- Availability: Hydrogen is readily available through the electrolysis of water and with a Peak Gas Generator it can be generated on demand.
- Hydrogen gas is already being used in the laboratory for a variety of purposes: It is the fuel gas for the most commonly used detectors (FID) and therefore already present in most GC Labs.





