Abstract
Due to rapid growth in the medical cannabis industry, demand is increasing for analysis of residual solvents in cannabis concentrates in order to protect consumer safety. This application note details a simple, fast test for common residual solvents using full evaporation technique headspace GC-FID and an Rxi®-624Sil MS column.

Introduction
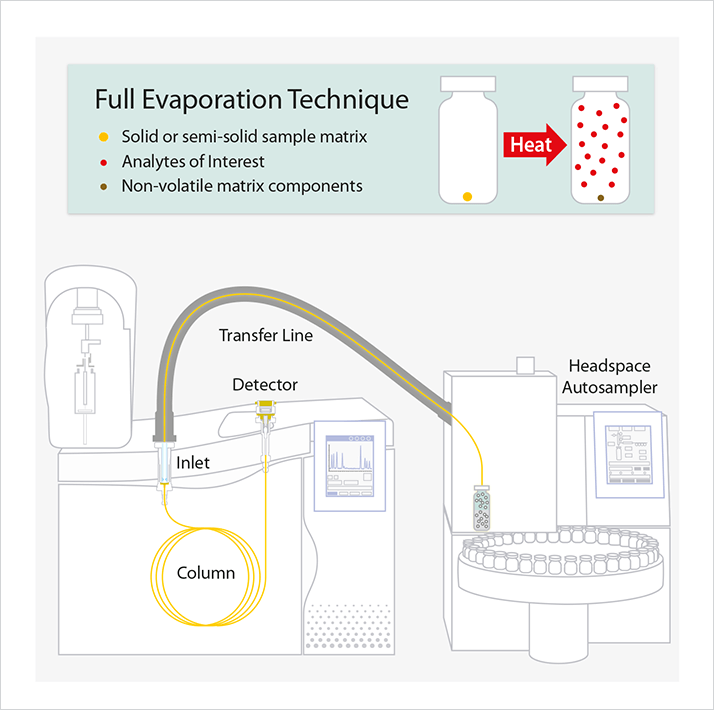
As the popularity of cannabis concentrates increases, consumer safety concerns are resulting in the establishment of new regulations to control the level of residual solvents in commercial cannabis concentrates. The State of Colorado, for example, published allowable concentrations of certain residual solvents in Rule R 712. This is because, although cannabis concentrates can be produced in numerous ways, one of the most common means of extracting therapeutic compounds, like tetrahydrocannabinol (THC), cannabidiol (CBD), and terpenes, from cannabis is through extraction with an organic solvent, such as butane. After the cannabinoids and terpenes are extracted from the plant material, the organic solvent is allowed to evaporate and then is purged off using heat and/or vacuum. These extraction solvents can be difficult to purge completely, so the finished product needs to be tested to ensure that residual solvents are only present at or below safe levels. For consumer safety, especially with medicinal products, accurate and comprehensive analysis of residual solvents is necessary for concentrates and extracts.Since residual solvents are extremely volatile, they cannot be analyzed by HPLC and lend themselves nicely to GC analysis. One of the most common and reliable ways to quantify residual solvents is through headspace gas chromatography–flame ionization detection (GC-FID). Headspace injection works by driving volatile compounds of interest from the sample into a gas phase in the headspace of the vial above the sample. An aliquot is then withdrawn from the headspace of the vial and analyzed by GC-FID in order to determine the volatile components of the sample. One approach for headspace GC-FID that is particularly useful for analyzing cannabis concentrates is the full evaporation technique (FET). FET sample preparation involves the use of a very small sample amount (e.g., 20–50 mg), which effectively creates a single-phase gas system in the headspace vial at equilibrium [1]. FET is ideal for difficult and varied matrices like cannabis concentrates because it eliminates matrix interferences that can cause inaccurate quantification, and it also has the advantages of little to no manual sample handling and a very small sample size. Additionally, high sensitivity can be achieved through the creation of a single-phase system in the headspace vial. Figure 1 illustrates the basic principle of headspace GC using the full evaporation technique.