Introduction
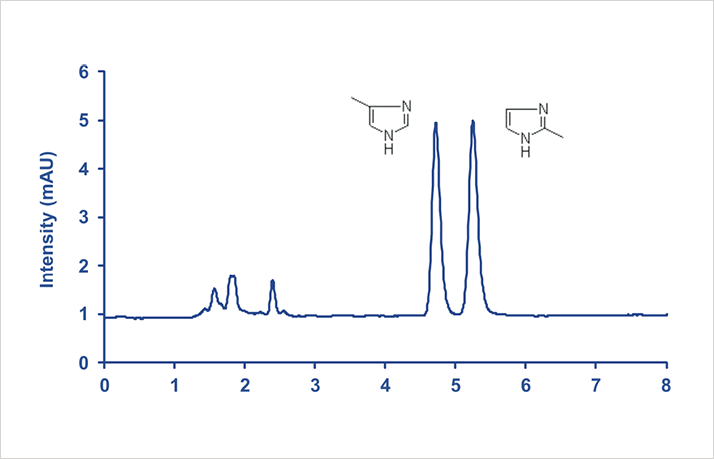
2- and 4-methylimidazole (2-MI and 4-MI), figure 1, are heterocyclic compounds derived from imidazole through substitution of the hydrogen in either position 2 or 4 by a methyl group. Both 2-MI and 4-MI are known animal carcinogens and they may form when carbohydrates and amino-containing compounds reacts during browning of food, but also in certain fermentation processes, and in types of caramel coloring produced with ammonia or ammonia and sulfites based processes. High levels of 4-MI has been found in many cola soft drinks including Coca-Cola, Pepsi-Cola, Diet Coke, and Diet Pepsi. Additionally, it has been found in roasted foods, grilled meats, and coffee. Dark beers and common cola soft drinks may contain more than 100 μg of 4-MI per 12-ounce canister.