Abstract
Protein biopharmaceuticals such as monoclonal antibodies and recombinant proteins are currently in widespread use for the treatment of various life-threatening diseases including cancer and autoimmune diseases. Protein therapeutics have a complexity far exceeding that of small molecule drugs, hence, unraveling this complexity represents an analytical challenge.
This Application Note demonstrates the potential of comprehensive two-dimensional liquid chromatography (LCxLC) using the Agilent 1290 Infinity 2D-LC Solution with DAD detection for the analysis of monoclonal antibody tryptic digests.

Introduction
Therapeutic macromolecules produced by recombinant DNA technology are becoming increasingly important in the treatment of various diseases. It is estimated that, at present, the global protein therapeutics market (monoclonal antibodies (mAb) and other recombinant proteins) represents approximately 20% of the total pharmaceutical market. Within the current decade, over 50% of new drug approvals will be biologics1. During the development and lifetime of these molecules, an in-depth characterization is required. This Application Note describes a peptide mapping method for identity and purity assessment of the monoclonal antibody trastuzumab. Trastuzumab, marketed as Herceptin, is a 150 kDa large molecule used in the treatment of HER2 positive metastatic breast cancer. Following trypsinolysis, over 100 peptides with varying physicochemical properties present in a wide dynamic concentration range are expected (Figure 1). This represents a complex analytical challenge, requiring the highest separation power. Comprehensive LCxLC is known to substantially increase the chromatographic resolution as long as the two dimensions are orthogonal and the separation obtained in the first dimension is maintained upon transfer to the second dimension2,3. This Application Note reports on the combination of strong cation exchange (SCX) and reversed-phase liquid chromatography (RPLC), two combinations shown to provide good orthogonality towards the analysis of peptides3. Fractions were transferred from the first to the second dimension using a dual loop interface maintaining the (first dimension) resolution. An Agilent 1290 Infinity 2D-LC Solution system was used. Some key results are highlighted and the performance of the system is demonstrated.
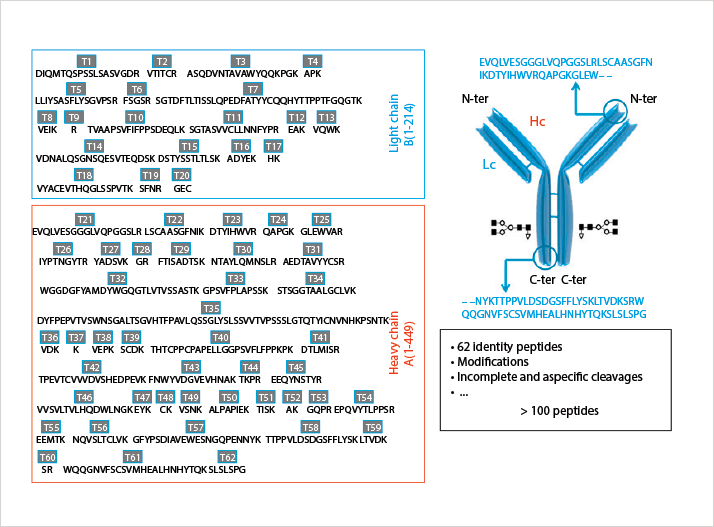 Figure 1. Structure and amino acid sequence of the protein. Identity peptides are labeled T1-T62.
Figure 1. Structure and amino acid sequence of the protein. Identity peptides are labeled T1-T62.