Introduction
The continuous influx of new synthetic drugs such as cannabis analogs into society is a major problem for law enforcement, forensic laboratories, and the medical community.1,2
Relatively simple organic transformations produce novel and licit psychotics that can elude detection by standard analytical methods.3,4 Detection and characterization of synthetic drugs is complicated by 1) the wide range of active ingredients and variety of botanical matrices, 2) the rate at which new drugs and blends appear on the market, 3) the fact that these synthetic drugs and metabolites are often not targeted during routine forensic analyses,5,6 and 4) these newly emerging compounds are typically not present in commercially available mass spectral libraries. High performance time-of-flight mass spectrometry is a practical choice for the analysis of these moving targets.
This application note shows the utility of high resolution mass spectrometry with soft ionization to facilitate identification of unknown compounds which were present in extracted residues from a confiscated pipe. When a compound is detected that is not matched sufficiently to a commercially available library, the electronic impact ionization (EI) spectrum becomes difficult to interpret without confirmation of the molecular ion. Accurate mass chemical ionization (HR-CI) results in preservation of molecular ions which is very important for structural elucidation.


Results and Discussions
A confiscated pipe was obtained from a collaborating forensic laboratory. Residues from the pipe were dissolved in organic solvent and analyzed by GC-HRT. The resulting analytical ion chromatogram (AIC) is shown in Figure 1.
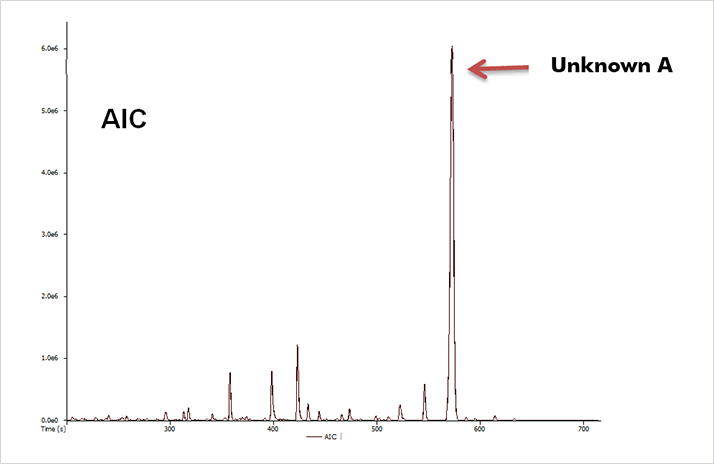 Figure 1. AIC obtained from EI analysis of pipe residue extract.
Figure 1. AIC obtained from EI analysis of pipe residue extract.The EI mass spectrum of the most abundant analyte detected ("Unknown A") in the residue is shown in Figure 2.
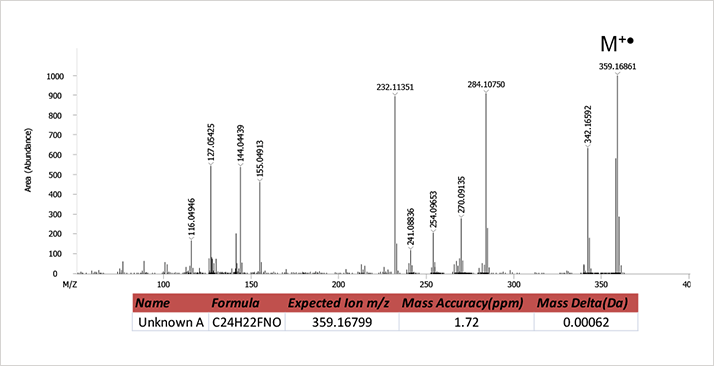 Figure 2. EI mass spectrum of unknown A.
Figure 2. EI mass spectrum of unknown A.A library search of the mass spectrum of Unknown A resulted in Lepenine as the number one hit with a spectral similarity of 546 out of a possible 1000points. The poor library match value and a quick inspection of the data suggests the unknown was not present in the commercially available libraries searched (NIST 2011 and Wiley 2009). The next step in the mass spectrometry work flow of unknown identification is to verify the molecular weight of the unknown compound. While the EI mass spectrum does contain a high mass ion at m/z 359.16861, there is no guarantee that this is the molecular ion peak without confirmation data from soft ionization massspectrometry. Therefore, a second analysis of residue extract was conducted using HR-CI with methane as the reagent gas. The resulting methane HR-CI mass spectrum is shown in Figure 3. This spectrum contains an intense protonated molecular ion at m/z360.17632 as well as a C2H5 adduct at 388.20798 confirming a molecular weight of 359 for the unknown and allows the analyst to proceed with structural elucidation of the obtained mass spectra. The excellent mass accuracies obtained using the Pegasus® GC-HRT allowed for confident formula assignments for molecular ions in both the EI and methane HR-CI spectra for the unknown. A formula search for the EIMS ion at m/z 359.16861 resulted in C24H22FNO (Mass delta = 0.00062, mass accuracy MA = 1.72 ppm). A similar search of the CI-MS ion at m/z 360.17632 resulted in the formula C24H23FNO (MA = 1.38 ppm). A web-based search of the formula C24H22FNO suggested the compound 1-(5-fluoropentyl)-3-(1-naphthoyl)indole, also known as AM-2201, as a likely candidate. A reference standard for this compound was purchased and analyzed to confirm that this was in fact the unknown analyte identified in the residue extract. The protonated chemical structure is shown as an inset in Figure 3.
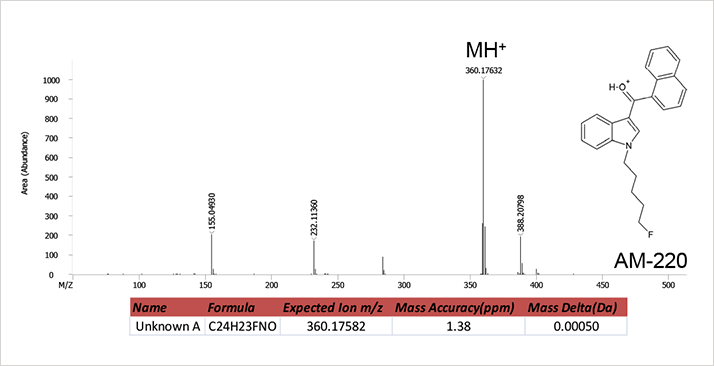 Figure 3. HR-CI mass spectrum of unknown A.
Figure 3. HR-CI mass spectrum of unknown A.




