Introduction
New roles and applications in the areas of science and technology are continuously being found for synthetic polymers. Specifically, the use of synthetic polymers in medicine is growing, as these polymers offer unique and versatile platforms for applications such as implants, medical devices, surgical adhesives, drug delivery vesicles, and injectable polymer-drug conjugates1,2. As the applications of synthetic polymers increases, there is a manifested need for methods to accurately and precisely characterize the materials

One of the most common and valuable tools employed for the analysis and characterization of polymers is size exclusion chromatography (SEC or gel permeation chromatography, GPC). The principle use of SEC, even a half-century after its inception, remains as determining the molar mass averages and distributions of natural and synthetic polymers through the application of calibration curves3. The applicability of SEC for synthetic polymers also extends into the realms of synthesis monitoring and oligomeric quantification. Synthesis monitoring using SEC not only allows for separation of polymeric material based on size but also provides information about the reactions, e.g., did the reaction go to completion, is the product uniform in terms of molar mass or size, did a byproduct form, etc. Oligomeric SEC plays an important role in the quantification of oligomeric content (i.e., low-molar mass species) of a polymer sample for the purposes of pre-manufacture notification (PMN) regulations for new chemical substances as well as import and export purposes3.The utility of SEC for synthesis monitoring and oligomeric analysis makes it an invaluable tool for characterizing synthetic polymeric material for use in medicine, as these materials require thorough characterization amongst other validations. Here we report on the use of an all-in-one dedicated GPC system, the EcoSEC® GPC System, to monitor the synthesis and to quantify the oligomeric content of two PEGylated synthetic polymers intended for use in medical applications.
Experimental Conditions
Sample analysis was performed on a system consisting of an EcoSEC GPC System (HLC-8320) equipped with a refractive index detector (RI). Separation of unfiltered 40 μL injections occurred over a column bank consisting of two 6.0mm ID × 15 cm, 3 μm particle size TSKgel® SuperH3000 columns (exclusion limit 60,000 g/mol) preceded by the appropriate guard column (Tosoh Bioscience). The solvent and mobile phase were tetrahydrofuran (THF) (Fisher Chemical) at a flow rate of 0.3 mL/min. Detector, pump oven, and column oven were maintained at 35 °C. Two PEGylated polymers and a starting material were analyzed: PEG-A, PEG-B and starting material C, respectively. For all chromatographic determinations, results are averages of three injections from two separate sample solutions. Sample solutions were prepared by diluting the sample (98% purity) with THF for a final sample concentration of approximately 10 to 15 mg/mL. Samples were shaken manually for a minute and allowed to sit for 3 hours before analysis was performed. Data was processed with the Eco- SEC GPC Workstation Software version 1.08.A calibration curve was created using PStQuick Kit-L polystyrene standards (Tosoh Bioscience) ranging in molar mass from 266 to 37,900 g/mol. Calibration curve data was fitted with a cubic function and error values were less than 5%.
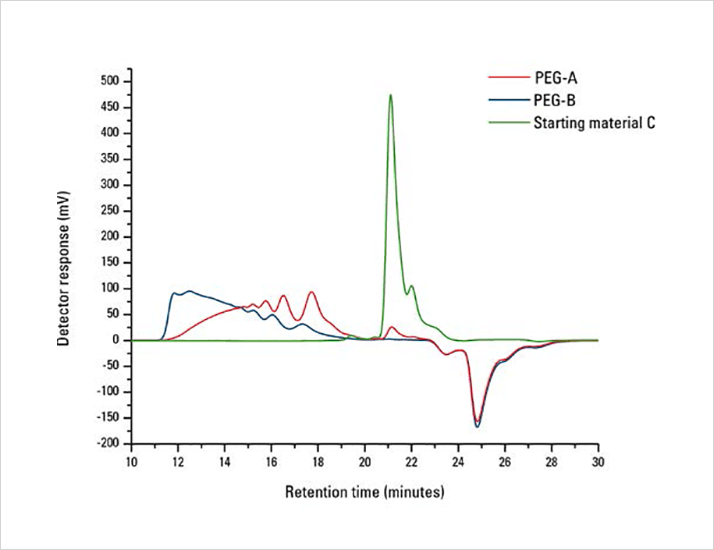 Figure 1. Synthesis monitoring by SEC. SEC elution profile of PEGA (red), PEG-B (blue), and starting mate rial C (green) as monitored by RI
Figure 1. Synthesis monitoring by SEC. SEC elution profile of PEGA (red), PEG-B (blue), and starting mate rial C (green) as monitored by RIResults and Discussion
As described above, an EcoSEC GPC System equipped with an internal dual-flow refr/media/virkliw0/a13i16a_analysis_pegylated_polymers_with_ecoseccleaned.pdfactive index (RI) detector was used for the characterization of two PEGylated polymers. The detector response of the RI detector was used to monitor the synthesis of the formation of the two PEGylated polymers and to compare the chromatograms of both materials to that of one of the starting materials. Additionally, a polystyrene relative calibration curve was used to determine the peak-average molar masses Mp and the oligomeric content of the two samples.





