Introduction
The antibody drug market has continued to expand in recent years, and antibody drugs held 6 of the top 10 blockbuster drug spots for 2015. The most promising antibody drug candidates for next-generation biopharmaceuticals are ADCs (antibody-drug conjugates). ADCs have a structure in which a cytotoxic drug is chemically bonded to an antibody (IgG).

Because there are numerous binding sites for a low-molecular weight drug on an antibody (Cys, Lys residues, etc.), heterogeneity arises with respect to the number of bonds and binding sites. It is necessary to study in detail the effect that this heterogeneity has on the medicinal effects and safety of ADCs. Since low-molecular drugs are more hydrophobic than monoclonal antibodies, differences in hydrophobicity arise when the number of bonded drug molecules differ. This property can be utilized to determine the drug-to-antibody ratio (DAR) by hydrophobic interaction chromatography (HIC). In this application note an ADC was analyzed using a TSKgel Butyl-NPR column.
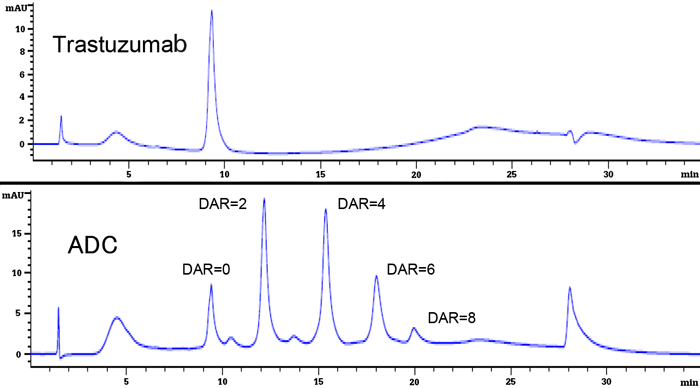 Analysis of Unconjugated Trastuzumab and Drug Conjugated Trastuzumab
Analysis of Unconjugated Trastuzumab and Drug Conjugated TrastuzumabTSKgel Butyl-NPR, the least hydrophobic of the TSKgel HIC columns, is the best choice for high speed separations with excellent recovery, even for more hydrophobic samples. The ultra-efficient 2.5 micron non-porous particles allow for fast and highly efficient separations. Thus, TSKgel Butyl-NPR columns (4.6 mm ID × 3.5 cm and 10 cm) are ideal for time critical QC analysis or sample-limited applications. An ADC (Trastuzumab-vcMMAE) in which an antineoplastic drug (monomethyl auristatin E, MMAE) is bonded via a linker to Trastuzumab was used. First results using common ammonium sulfate gradient elution conditions showed that the ADC could not be suitably eluted. Hence, an organic solvent (2-propanol) was added to eluent B, and by optimizing the organic solvent concentration, peaks exhibiting different DARs (DAR = 0 to 8) could be well separated (to confirm the DAR, each peak was fractionated and attributed by LC-MS/MS).





