Abstract
In this paper the importance of reagent water quality for toxic element environmental analyses is discussed, and the suitability of fresh ultrapure water produced using Merck water purification systems for ICP-OES and ICP-MS trace element analyses in environmental laboratories is demonstrated.

In this paper the importance of reagent water quality for toxic element environmental analyses is discussed, and the suitability of fresh ultrapure water produced using MilliporeSigma water purification systems for ICP-OES and ICP-MS trace element analyses in environmental laboratories is demonstrated.

Introduction and Water Quality Requirements
Dramatic improvement in the sensitivity of analytical instruments over the last decades has changed our understanding of environmental contamination and hazardous effects of metals such as Be, Cr, Mn, Fe, Ni, Cu, Zn, As, Cd, Sb, Ba, Hg, Tl, and Pb. This has resulted in a number of regulations and guidelines that establish the maximum acceptable or recommendable concentrations of toxic metals in drinking water,1 marine water,2 and wastewater.3 The requirements instituted by authorities consequently have resulted in a growing need for toxic metal monitoring in environmental laboratories where spectrometry techniques are standard instrumentation recommended for the determination of trace elements.4,5 The preponderant role of ICP-MS and ICP-OES in the detection of traces of toxic metallic elements in environmental analyses of water and soil has led to higher quality requirements for ultrapure water, which is the most frequently used reagent in ICP-MS and ICP-OES analyses. In particular, ultrapure water is used as the reagent blank, for sample and standard preparation, and for instrument and sample container cleaning (Figure 1). Therefore, the ultrapure water must be free of metals to preserve analytical instruments from contamination and to avoid interferences with analyzed elements, in order to ensure the accuracy and precision of measurements.
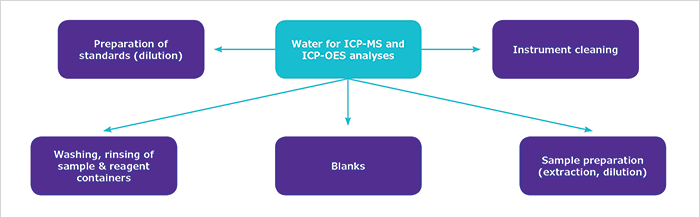 Figure 1. Different types of uses of ultrapure water in ICP-MS and ICP-OES analyses.
Figure 1. Different types of uses of ultrapure water in ICP-MS and ICP-OES analyses.Results and Discussion
To benefit fully from modern ICP-OES and ICP-MS instrumentation, ultrapure water of very good quality is required. Indeed, any contamination coming from laboratory reagents will increase background equivalent concentration (BEC) and detection limit, resulting in poorer performance of the technique. Therefore, the suitability of reagent water used in all steps of ICP-MS or ICP-OES analyses is defined by the general rule that the measured element should not be detectable in the blank, or if it is detected, its BEC should be negligible relative to the desired analytical range. In environmental analyses, elements in water samples are usually analyzed at μg/L (ppb) analytical range6 and in soil samples, at mg/L (ppm) range.7 To ensure the success of experiments in the ppb-ppm range, it is desirable that BEC values of target elements do not exceed ppt or sub-ppt range. Moreover, as LOD (Limit of Detection) is separately specified in certain analyses,1 in addition to a negligible level of contamination, the usage of ultrapure water of consistent quality is critical.





