Analytical laboratories face more challenges and regulations than ever before as accreditation bodies issue increasing numbers of guidelines; and regulatory agencies increase the number of analytes that need to be reported while the levels of detection required decrease. A lot of time, effort and money is invested in deciphering the data and determining its validity and accuracy. Often terms which describe data are used incorrectly or interchangeably to try to validate a data set or methodology (i.e. error vs. uncertainty, precision vs. accuracy, etc.). One of the first steps to understanding and validating data is the proper application of appropriate statistics and the understanding of the use and terminology of analytical processes.

True Value, Accuracy and Precision
All analytical laboratories pursue ‘good’ data and ‘true’ values. The reality is that true values are never absolute. The nature of a true value is that it, in itself, contains uncertainty and error which make them somewhat indeterminate. True values are obtained by perfect and error-free measurements which do not exist in reality. Instead, the expected, specified or theoretical value becomes the accepted true value. Analysts then compare the observed or measured values against that accepted true value to determine accuracy or ‘trueness’ of the data set. Often accuracy and precision are used in the same context when discussing data quality. In reality, accuracy and precision are very different assessments of data and the acquisition process. Accuracy is the measurement of individual or groups of data points in relationship to the ‘true’ value. In essence, accuracy is how close your data gets to the target and is often expressed as either a form of numerical or percent difference of the observed result and the target or ‘true’ value.
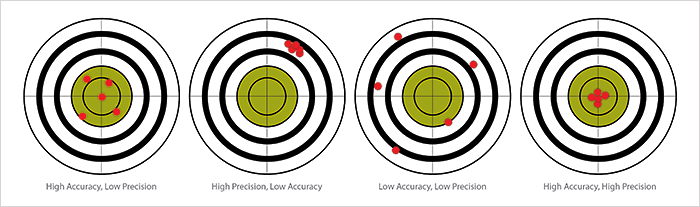 Figure 1. Representations of Accuracy and Precision.
Figure 1. Representations of Accuracy and Precision.>> Download the full Application Note as PDF
>> Company website
Contact Name: Amy Evans
Contact Phone Number: 1-732-549-7144





