Overview
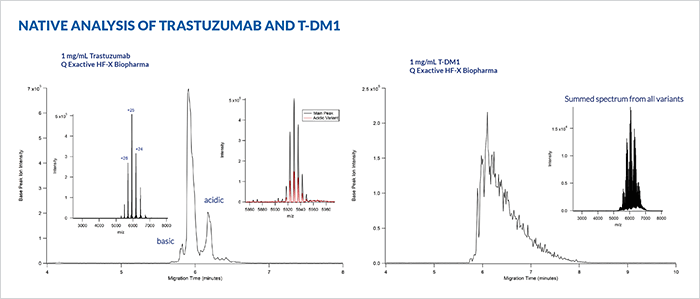
The characterization of intact monoclonal antibodies by mass spectrometry is challenging due to the size and complexity of the molecules. The ability to separate charge variants in a separation that is directly coupled to the mass spectrometer greatly improves the MS analysis by minimizing spectral overlap of similar variants, while also providing a charge variant separation which aids in identification of the species. We have previously demonstrated the ability to do such an analysis using microfluidic capillary electrophoresis – ESI-MS under “near native conditions”. This current work extends that capability by operating under fully native conditions which maintain the folded structure of the molecules both during the CE separation and through the ESI transition into the gas phase.

Methods
All work was performed using a commercially available microfluidic CE-ESI system (ZipChip®, 908 Devices Inc.), attached to an orbitrap mass spectrometer (Exactive EMR or Q Exactive HF-X Biopharma, ThermoFisher). The microfluidic devices utilized a covalently attached, neutral polymer surface coating to prevent analyte interactions and suppress electroosmotic flow. An optimal background electrolyte (BGE) was developed to maintain fully native conditions while maximizing the resolving power of the separation and the sensitivity of the ESI-MS. The method was initially tested with the NIST monoclonal antibody reference material (RM 8671, purchased from NIST), along with approved drug molecules trastuzumab, and trastuzumab emtansine (T-DM1) which were kindly provided by collaborators. All samples were diluted directly from formulation to a concentration of 1 mg/mL. No other sample prep was done. All samples were run with the same separation method. Data were processed using Biopharmafinder 1.0 (ThermoFisher).