CDS Analytical’s 7000C Purge and Trap concentrator designed for PAL System fully automates Purge and Trap for the trace measurement of purgeable volatile organic compounds (VOCs) in water, compliant with the official International Standard Organization method DIN-EN ISO 15009, US EPA method 500 and 8000 series for VOCs in water. In this application note data is presented that the 7000C/PAL System exceeds the performance criteria set of EPA Method 8260C.
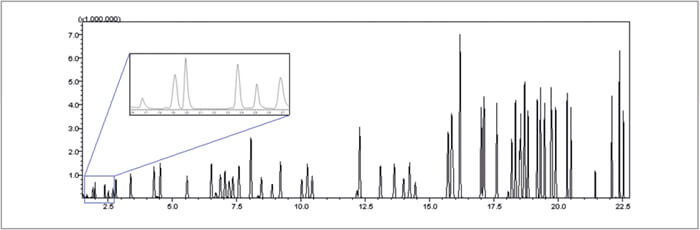
Figure 1 is the Total Ion Chromatogram (TIC) of a 200 μg/L calibration standard with internal standard and surrogates at total of 64 compounds mix. All of the analytes are adequately resolved chromatographically. The chromatogram of the six gases is enlarged in the insert in order to show the excellent separation and peak shapes.

The Retention Time (RT), Average Relative Response Factors (Avg RRF), Percent Relative Standard Deviation (% RSD) of the initial calibration, Method Detection Limits (MDL), along with method accuracy as Percent Recovery (% Rec) and as % RSD are obtained from 0.5 μg/L to 200 μg/L calibration standard, and all analytes exceed the EPA 8260C method requirements. The detailed data for 64 compounds is available in the full length application note.
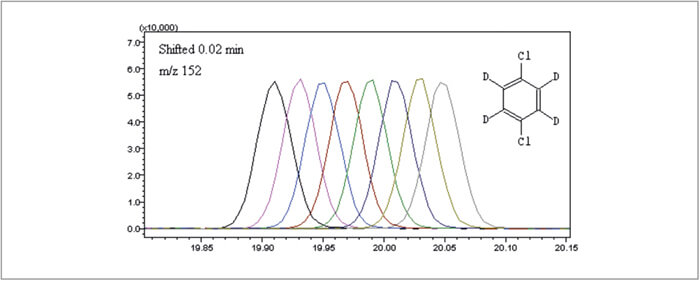
The Internal Standard Module precisely delivered 1 μL of the pre-mixed internal standard solution to each sample. The reproducibility data from 8 runs is shown in Table 1. An excellent RDS < 2.4% is reported. Figure 2 is the time-shifted overlap of 8 1,4-Dichlorobenzene-d4 runs using the internal standard module.
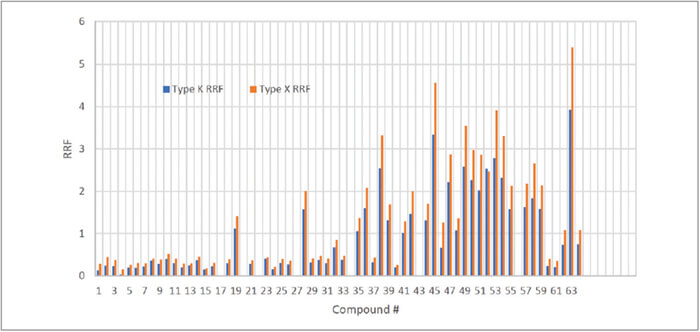
Although all the data above was collected in a 7000C with a CDS proprietary type X trap installed, a comparison test was performed against the regular type K (Vocarb 3000) trap in the same system. Figure 3 showed the RRF comparison between the two traps for all the 8260C compounds, where an average of 30% increase in RRF from type X trap is observed. Among all the 8260C compounds, 2,2-dichloropropane, which is commonly considered as a testing compounds to trace the active site in the flow path, has 48% increase in RRF from using the Type X trap.





