Environmental conditions play a critical role in polymer lifetime, and traditional degradation studies take time ranging from hours to days due to limited light intensity. CDS’s Photoprobe uses freespace focusing technology and improves the light intensity to 800 mW/mm2 from 260 nm to 400 nm wavelength, which reduces the time on weather-induced degradation studies down to minutes.
A CDS 6200 Pyroprobe equipped with Drop-In-Sample Chamber (DISC) and Photoprobe was used, and an autosampler module was installed to automate the weathering-pyrolysis sequence. 11 μg of polystyrene was irradiated in the DISC with the presence of air as reactant gas at a flow rate of 10 ml/min. The volatiles generated from the photoreaction were trapped on the analytical trap, and then desorbed to the GC-MS after the photoreaction is completed. The remaining polymer was pyrolyzed at 600°C as the last step. A DISC quartz tube was used as the sample vessel.
CDS Pyoprobe Setting:
Method 1 - Weathering
DISC: 60°C
Photoprobe: 60% Intensity
UV irradiation: 5 min
Reactant Gas: Air 10mL/min
Trap Rest: 40°C
Trap Final: 300°C 3 min
Trap Sorbent: Tenax
Method 2- Pyrolysis
DISC: 600°C 30seconds
Interface: 300°C
Valve Oven: 300°C
Transfer Line: 300°C
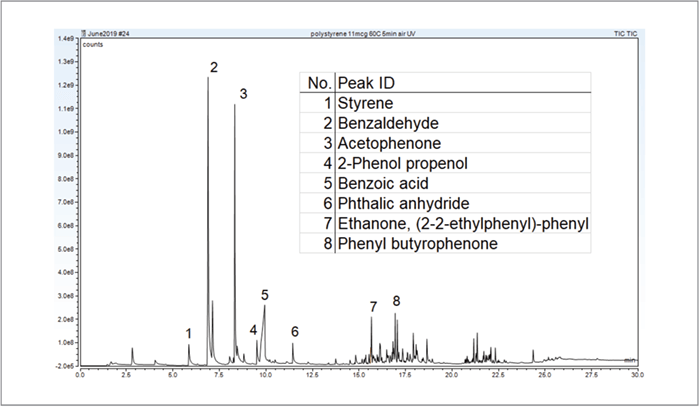
Weathering studies of polymers with the Photoprobe took minimal time – on the scale of minutes compared to hours with traditional techniques. Polystyrene under UV irradiation and an air atmosphere at a 60°C set-point produces many UV thermal oxidative degradation products, ranging from volatile to non-volatile components. The resulting chromatogram is shown in Figure 1.
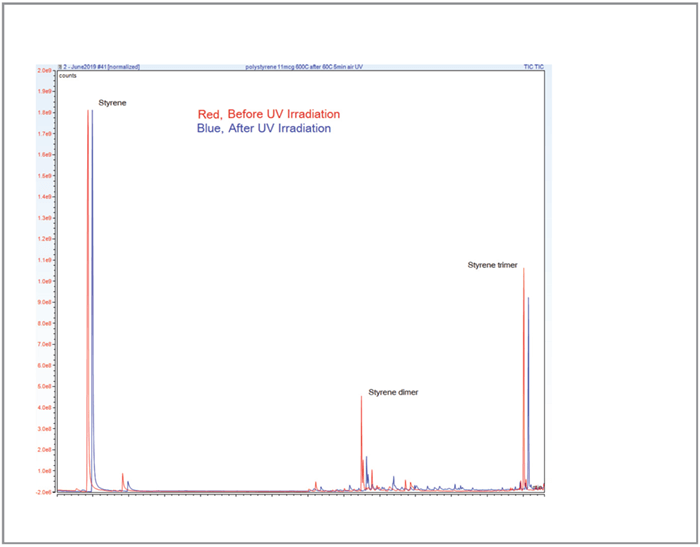
Six replicates of the volatiles from weathering polystyrene provided area ratio RSDs ≤5.25 percent for Benzaldehyde, Acetophenone and Styrene (Table 1). After this degradation analysis, the remaining degraded polymer was automatically pyrolyzed and studied with a second GC-MS run (Figure 2). Six replicates of the pyrolysis of weathered polystyrene produced an area ratio RSD of 3 percent.
In addition to analytical pyrolysis, the Photoprobe, the newest member of the CDS Pyroprobe family, can perform quantitative online weathering studies within minutes.