In modern organic and medicinal chemistry, fluorine is used to enhance the chemical properties of molecules in many desirable ways. When substituted for hydrogen, the increased stability of the C-F bond can delay the metabolism of the molecule and forbid peroxide formation. As well, its heightened lipophilicity has a direct positive effect on the molecule’s bioavailability [1]. Consequently, it is estimated that more than 20% of commercial pharmaceutical APIs, 30% of the top 15 prescribed pharmaceuticals, and 30% of agrochemicals contain at least one fluorine atom [2,3]. While advances have been made to selectively introduce fluorine in drug development, more research is required to understand the direct effect of these additions on specific chemical structures. A comprehensive understanding of this can increase the likelihood of developing a successful compound and reduce development time [4].
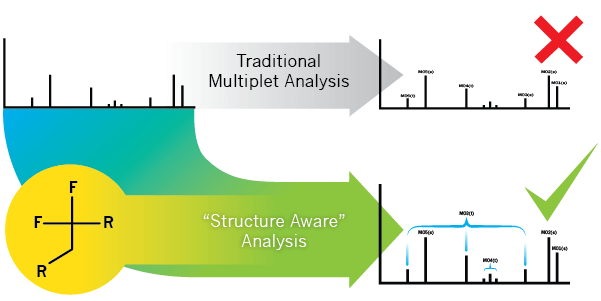
A challenge in the development of fluorine chemistry is the potential difficulty of interpreting 13C NMR spectra of fluorinated organic compounds. This is because 13C spectra are commonly recorded using only 1H broadband decoupling and the 13C-19F couplings are still present. The C-F coupling constants can be very large (up to 250 Hz or more), which may result in multiplets severely overlapping with other peaks in the spectrum. Additionally, since 13C spectra usually have low signal to noise ratios it is frequent for the lower outer part of a multiplet to lie below the noise level and not be visible. To mitigate this, it is possible to record 13C spectra broadband decoupled from both 1H and 19F but it requires specialized NMR probes and decoupling techniques. Moreover the very broad range of 19F chemical shifts could pose a danger of damage to the probe due to the excessive power that would be required. Consequently, this approach is not considered practical for routine use. As an alternative, more consistent spectral analysis methods have the potential to reduce costs, error and expedite analysis time.
Here we present an analysis method that reliably peak-picks and identifies multiplets in the 13C spectra of organic compounds based on the accurate prediction of C-F multiplets and matching with the experimental spectrum. We show that regardless of whether the final results contain multiple, overlapping multiplets, the expected carbon resonances are reliably identified and assigned for each spectrum.