Introduction
During the last decade, the production and use of engineered nanomaterials (ENMs) have experienced a drastic increase, resulting in a potential risk of their release into the environment. Therefore, the study of their impact on the environment becomes crucial. The appropriate ecological risk assessment and management of ENMs in the environment requires quantitative measurements of both exposure and effects1 that should, ideally, be performed by in situ analysis and give physicochemical characterization. However, most analytical techniques are not suitable for environmental matrices since nanoparticle concentrations are typically very low2.
Some studies on the persistence, aggregation and dissolution of metal nanoparticles in natural freshwaters and synthetic complex waters were recently published3-7. Historically, particle size has been measured by dispersive light scatter (DLS) and tunneling electron microscopy (TEM), while dissolved content has been measured by ultrafiltration. These common techniques have known limitations for measuring low concentrations in the presence of colloidal species in complex waters.


Alternatively, single particle inductively coupled plasma mass spectrometry (SP-ICP-MS) has been found to be a promising technique for detecting and characterizing metal nanoparticles at very low concentrations8-10. SP-ICP-MS is fast and efficient and can provide more information than other currently available techniques. It can lead to the determination of particle size distribution, particle number concentration, and the proportion of dissolved metal. Moreover, it can distinguish between particles of different elements. The principle of SP-ICP-MS is based on the measurement of the signal intensity produced by a single particle. The suspension of nanoparticles must be sufficiently diluted to make sure that only a single particle reaches the plasma at a time, where it is atomized and ionized, producing a signal of relatively high intensity which is measured as one pulse. If the suspension contains dissolved metal of the same element as in the particles, a constant, continuous signal of that element will be produced as a result of its homogeneous distribution. The recorded signal intensity as a function of time can be processed using a theoretical approach first developed by Duegeldre et al.11-15 for natural metal colloids and then supported by other authors such as Laborda et al.16, 17.Ag, ZnO and TiO2 are among the most frequently studied nanoparticles18. In the case of Ag, this is most probably due to the fact that nanosilver is among the most common nanomaterials found in consumer products and tends to release free Ag+, especially at low concentration19. The aim of this work is to investigate the efficiency of SP-ICP-MS for the detection and characterization of metal nanoparticles in environmental waters where they can be involved in various physicochemical processes as shown by Figure 1. Dissolved silver, including released free ions (Figure 1C) and soluble complexes (Figure 1E), can easily and instantly be measured by SP-ICP-MS. These dissolved species can also be determined by ultrafiltration followed by total metal quantification using ICP-MS or AAS, but this procedure is time consuming since it requires the pre-equilibration of the membrane for at least three cycles of centrifugation, generally 20 min each19. Aggregates (Figure 1B) and remaining stable silver nanoparticles (Figure 1G) can be counted and measured by other commonly used techniques (DLS, NTA, TEM) but SP-ICP-MS is the only method that can distinguish between nAg and other colloids in surface water.
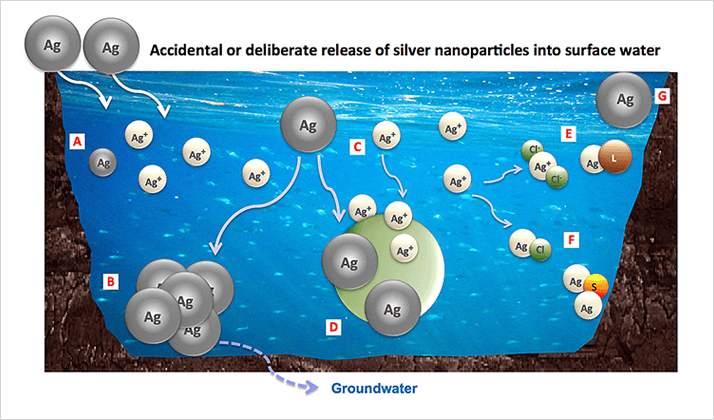 Figure 1. Possible fates of silver nanoparticles in surface waters: (A) Dissolution process leading to free ions release and smaller particles; (B) Aggregation into larger particles, which may settle out of the water, depending on the aggregate size; (C, D) Adsorption of released Ag+ and nAg, respectively, onto other solids present in the water; (E) Formation of soluble complexes; (F) Reaction with other components in the water, which may result in precipitation; (G) nAg remaining stable.
Figure 1. Possible fates of silver nanoparticles in surface waters: (A) Dissolution process leading to free ions release and smaller particles; (B) Aggregation into larger particles, which may settle out of the water, depending on the aggregate size; (C, D) Adsorption of released Ag+ and nAg, respectively, onto other solids present in the water; (E) Formation of soluble complexes; (F) Reaction with other components in the water, which may result in precipitation; (G) nAg remaining stable.




