Abstract
Analysis of drug panels in urine samples can be challenging, and the trend towards larger panels including multiple drug classes compounds the issues faced during method development.
This white paper examines a number of aspects of sample preparation, and their impact on the success of subsequent LC-MS/MS analysis of broad urine panels.
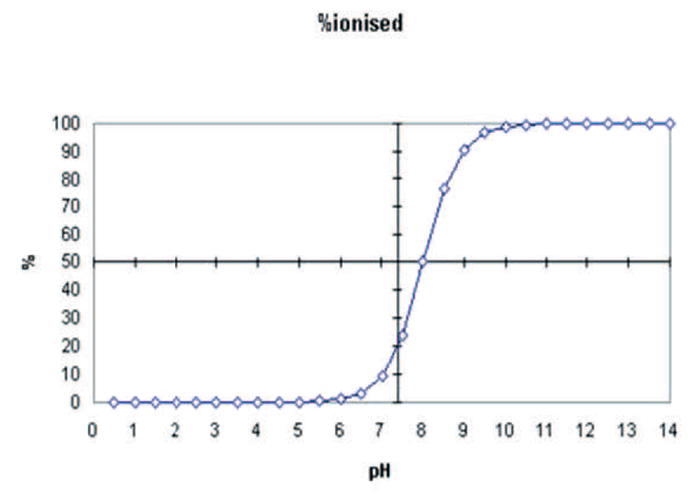
Section 1 examines the applicability of various sample preparation techniques: supported liquid extraction, reverse phase SPE and mixed-mode SPE, to the various classes of drugs extracted. In addition, hydrolysis approaches: enzyme type and protocol used (time, temperature), are compared.
Mixed-mode reverse phase/cation exchange SPE is widely used for extraction of basic drug classes from urine, but the inclusion of drugs and metabolites that exhibit ‘non-typical’ functionality within urine panels can be problematic. Section 2 examines the impact of various parameters (interference wash strength, elution solvent composition) on analyte retention, elution and extract cleanliness with particular focus on zwitterionic (gabapentin, pregabalin) and non-ionic (carisoprodol, meprobamate) drugs.

Section 1.
Practical Considerations for LC/MS Method Development of a Comprehensive Urine Pain Panel By Stephanie J. Marin Ph.D., Dan Menasco Ph.D., Jillian Neifeld and Elena Gairloch. Urine drug testing to support pain management is a mainstay of the clinical toxicology laboratory. Reduced reimbursement has continued to put increasing pressure on laboratories. Many toxicology labs are moving to larger drug panels to increase throughput and efficiency, while reducing turnaround time and cost. LC-MS methods with 50 or more drugs and metabolites are common. While “dilute and shoot” (D&S) methods are easy and affordable, they can result in shortened LC column lifetimes and increased MS instrumentation downtime. Matrix effects can also affect sensitivity and overall method performance. Clean-up of hydrolyzed urine specimens can reduce matrix effects, increase LC column lifetimes, and keep MS instrumentation cleaner. The result is an overall increase in efficiency and productivity because of reduced downtime. Samples can also be concentrated, resulting in improved sensitivity. Here, we present sample preparation considerations for a panel of 56 drug analytes by three different sample preparation methods: supported liquid extraction (ISOLUTE® SLE+), polymeric reverse phase solid phase extraction SPE (EVOLUTE® EXPRESS ABN), and mixed-mode polymeric reverse phase strong cation exchange SPE (EVOLUTE® EXPRESS CX). Details of the sample preparation methods used are shown on page 5.
 Acid Analyte Plot
Acid Analyte Plot




