Introduction
Detailed hydrocarbon analysis (DHA) is a separation technique used by a variety of laboratories involved in the petrochemical industry for analysis and identification of individual components as well as for bulk hydrocarbon characterisation of a particular sample. Bulk analysis looks at gasoline composition in terms of PONA components (Paraffins, Olefins, Naphthalenes and Aromatics) and other fuels in the C1-C13 range since this gives an indication of overall quality of the sample.

Results and Discussion
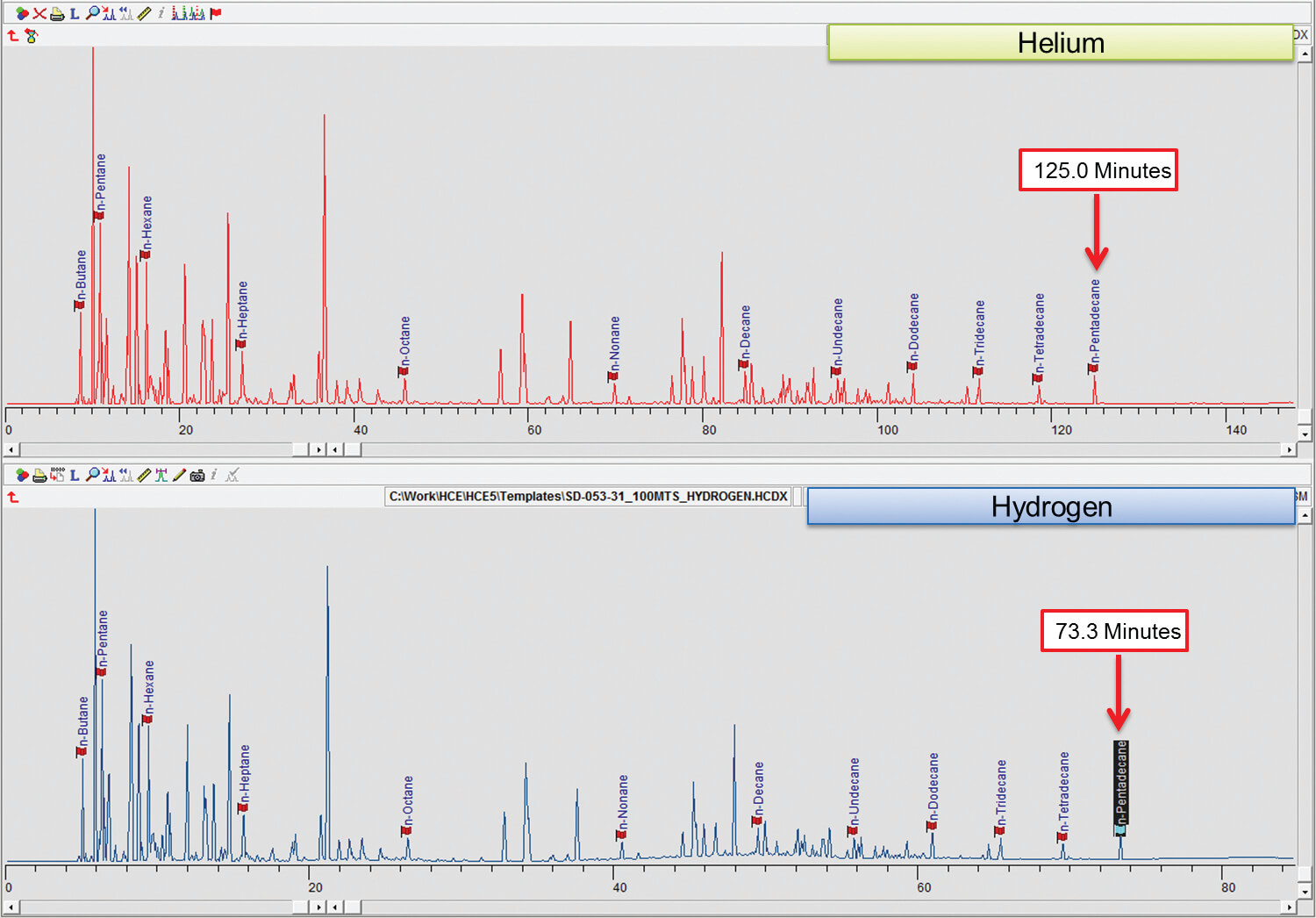
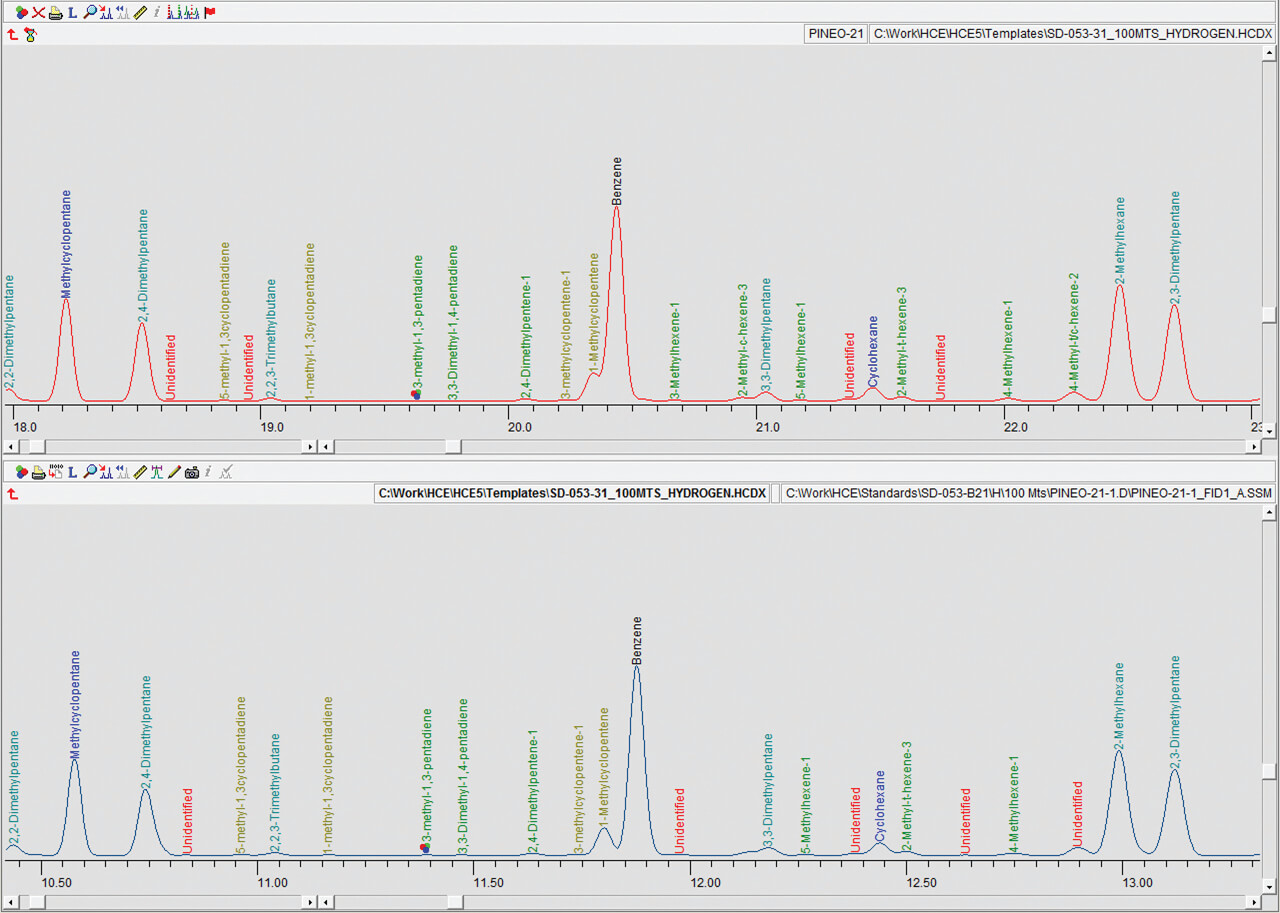
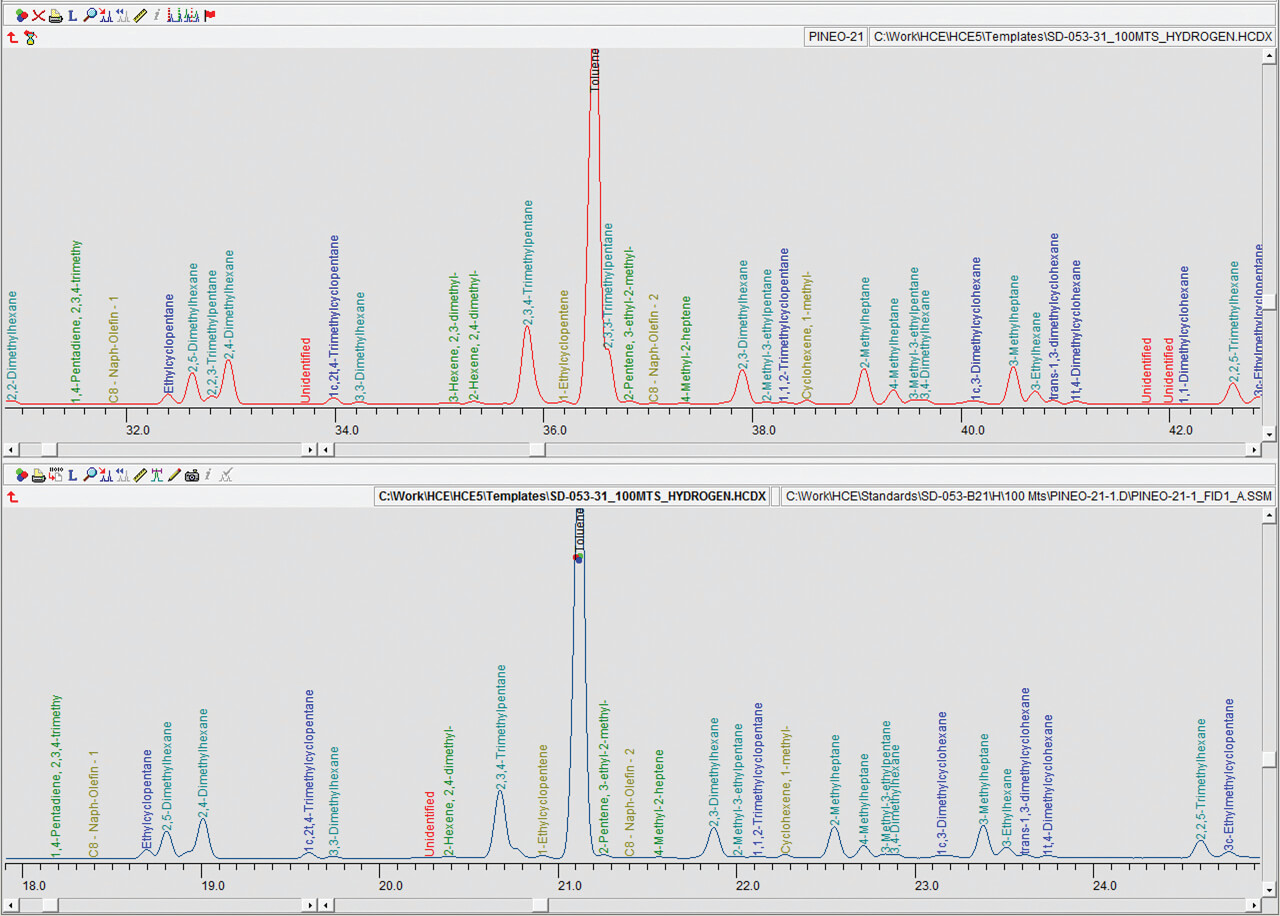
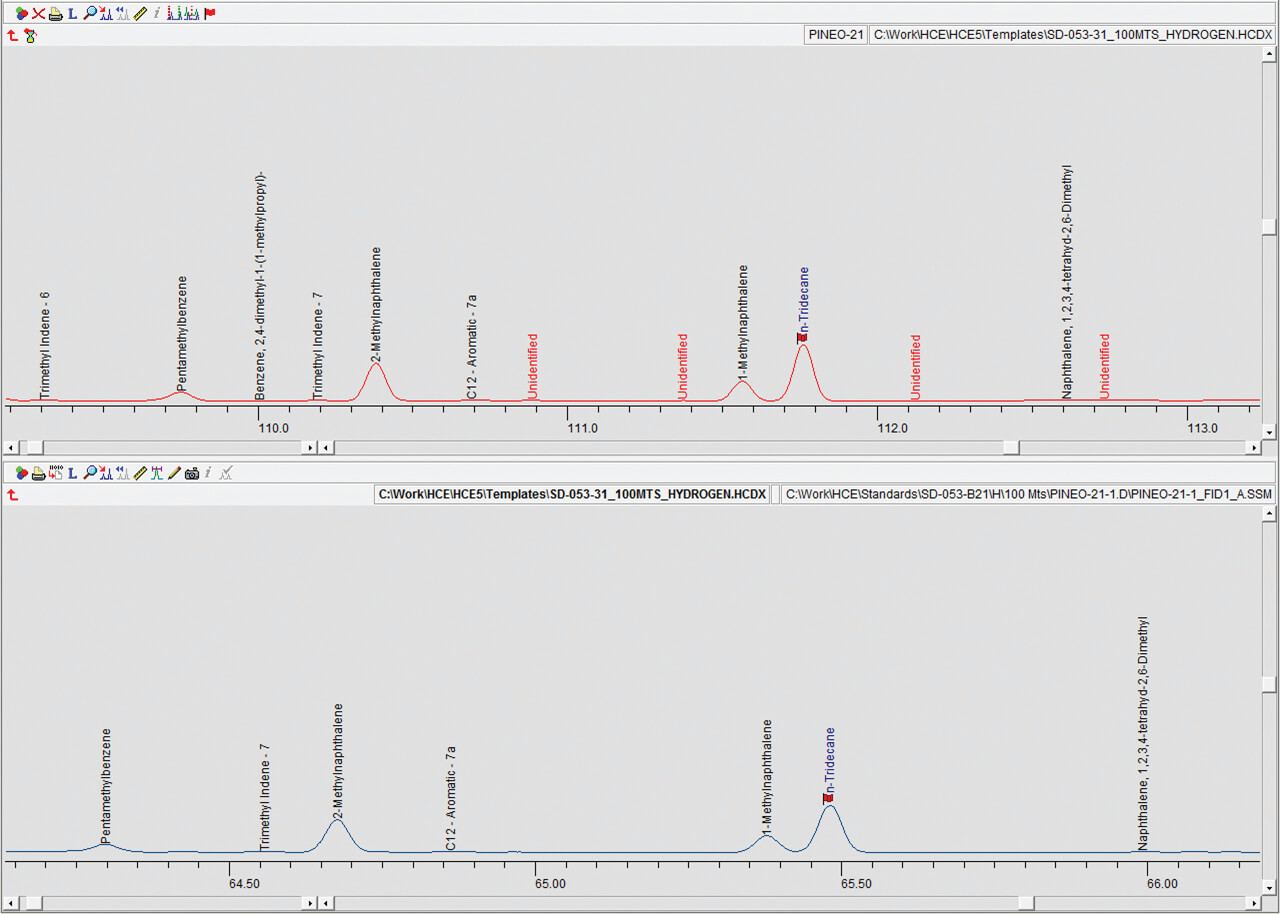
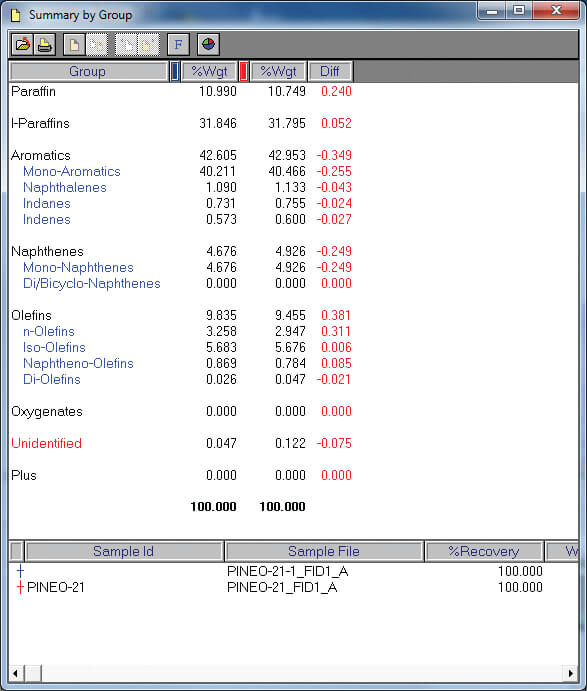
Detailed hydrocarbon analysis of gasoline showed that the elution time of the last compound in the mixture, n-Pentadecane, could be reduced from 125 minutes to less than 74 minutes by switching carrier gas from helium to hydrogen (figure 1). Despite the difference in analysis times, the PONA analysis showed that quantitative differences were not significantly different when using either carrier gas (table 1). Despite the much higher carrier gas flow rates when using hydrogen carrier gas, critical separations were still achieved in most cases and in certain cases were even improved. Separation of 1-methylcyclopentene and benzene, which is highly regulated analysis because of the importance of the benzene fraction, was actually improved when using hydrogen carrier gas despite the quicker elution times of the compounds with hydrogen as a carrier gas (figure 2). Separation of Toluene and 2,3,3-Trimethylpentane was achieved using helium whereas with hydrogen the two compounds co-eluted (figure 3. To separate these two compounds using hydrogen carrier gas some improvements to the method would need to be made. Separation of Tridecane and 1-methylnaphthalene was achieved equally well using both carrier gases (figure 4).The results of the DHA show that the use of hydrogen as a carrier gas, following ASTM D6729 appendix 2 methodology can vastly reduce analysis times for gasoline analysis whilst providing the necessary resolution required for separations of critical components.