Summary
This application note describes a Solid Phase Extraction (SPE) protocol for the extraction of aflatoxin M1 (AM1) internally standardized with aflatoxin B2 (AB2) from infant formula using ISOLUTE® Myco SPE columns with LC MS/MS.
 Application Note AN807
Application Note AN807Introduction
Mycotoxins are toxic metabolites produced by fungal molds on food crops. Regulation and legislation for testing of mycotoxin contamination has established which mycotoxins are prevalent on a wide variety of food crops. This application note describes an SPE protocol appropriate for LC-MS/MS analysis of aflatoxin M1 found in infant formula. The method described in this application note achieves high recoveries of aflatoxin M1 from infant formula with %RSDs and LOQs that all meet the requirements set in European regulations for its measurement. ISOLUTE Myco solid phase extraction columns provide robust, reliable sample preparation for multiple mycotoxin classes from a wide range of foodstuffs. Using a single, easy to use sample preparation product, along with optimized matrix specific application notes, scientists can prepare diverse food/crop samples for analysis by LC-MS/MS.
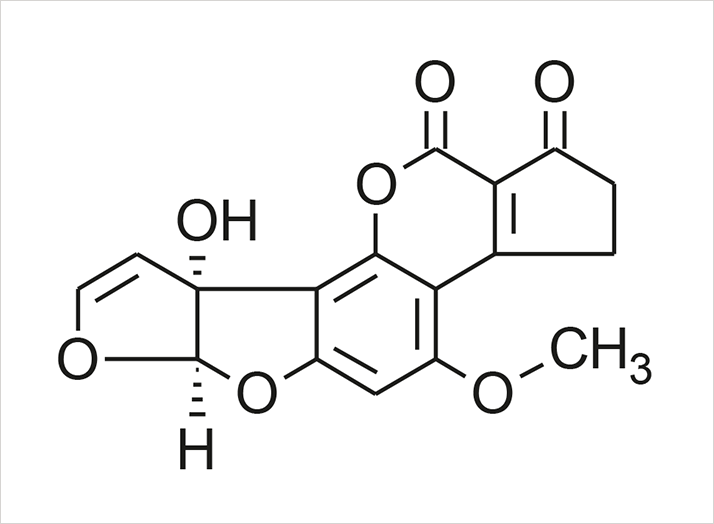 Figure 1. Structure of Aflotoxin M1
Figure 1. Structure of Aflotoxin M1Analytes
Aflatoxin M1, aflatoxin B2 (internal standard).
Sample Preparation Procedure
Format:
ISOLUTE® Myco 60 mg/3 mL (Tabless), part number 150-0006-BG Sample Pre-treatment:
- Sample processing: reconstitute the infant formula according to the manufacturer’s recommendations using 1% formic acid (aq) as the solvent. Add a small volume of AB2 at an appropriate concentration (e.g. 18 μL x 100 ng mL-1 AB2 / 36 mL formula = 50 ng L-1)
- Extraction: shake the reconstituted formula vigorously by hand for 30 seconds. Place the sample tube in an ultrasonic water bath and sonicate for 20 minutes. Centrifuge the sample tube at 4000 g for 10 minutes.
- Work-up: Spoon off and discard the upper cream layer.





