Abstract
A new and broad product line for the analysis of peptides and proteins by reversed-phase high performance liquid chromatography (RP-HPLC) is introduced under the name BIOshell Fused-Core U/HPLC columns. As the Ascentis® Express column line for the analysis of small molecular mass compounds, BIOshell columns outperform fully porous silica-based columns of the same particle size in terms of one or more important chromatographic attributes; such as efficiency, resolution, analysis time (speed) and back pressure. By providing faster peptide and protein liquid chromatography (FP2LC), the benefits of porous-shell columns are now also available to scientists who are tasked to advance our knowledge about the living world.

Background and Characteristics
The development of Fused-Core U/HPLC columns is based on the pellicular silica technology developed by J. J. Kirkland at E. I. du Pont de Nemours and Company in the late 1960s during the formative years of HPLC, and applied in this century to create very efficient particles in the range of 2.7 to 5 micron.1,2 The latest innovations derived from this technology are now available as BIOshell Fused- Core columns for faster protein and peptide liquid chromatography (FP2LC) separations. BIOshell columns for peptide analysis are either packed with 2.7 or 5 micron particles containing 160 Å pores and bonded with a C18 alkyl or alkyl cyano functionality, while protein separations are best performed with 3.4 micron particles containing 400 Å pores bonded with C4 alkyl functional groups. The complete list of available BIOshell columns is provided at the end of this article.
In 2007 Supelco introduced the groundbreaking Ascentis Express Fused-Core column line for the analysis of compounds with low molecular mass. In the succeeding years it has become clear that (a) columns packed with 2.7 micron, 90 Å pore size, porous-shell particles provide a dramatic and unexpected improvement in column efficiency, rivaling the efficiency of columns packed with fully porous 1.7 micron particles, and (b) a paradigm shift had to take place in the minds of theoreticians to adjust the theory of chromatographic band broadening to account for column efficiencies that were, until then, considered outside the realm of possibility.3,4
Like the particles in Ascentis Express columns, the particles in BIOshell columns are composed of a spherical solid glass core surrounded by a thin layer of nano-size silica particles. Table 1 summarizes the characteristics and maximum operating conditions of the new BIOshell porous-shell columns.
As shown in Table 1, to accommodate the larger size of biopolymers, the particles in BIOshell columns either feature160 Å pores for unhindered access by peptides up to a mass of about 20 kDa, or 400 Å pores for proteins with a molecular mass up to about 500 kDa. Note that the thickness of the porous shell varies as does the percentage of the surface area when compared to a fully porous particle of the same size. Thus, the 0.2 micron shell for the 3.4 micron particles results in a surface area that is 31% of a fully porous particle of that size. Similarly, the 0.5 micron shell for the 2.7 micron particle provides 75% of the maximum surface area, while the 0.6 micron shell for the nominally 5 micron but actually 4.7 micron particles contains 59% of the surface area of a fully porous particle. Although the loss of surface area may seem a cause of concern, Unger et al., first demonstrated that even nonporous matrices provide sufficient retention for larger biomolecules, while Gritti and Guiochon showed that the capacity of core-shell particles is not substantially reduced even for large biopolymers, a conclusion that was recently confirmed in a paper from Guillaume et al., who found that the loading capacity of a wide pore core-shell column was 2–3 fold less than that of a fully porous column.5-7
Table 1 also shows the bonded phase characteristics, recommended pH range, and the highest values for back pressure and temperature at which each particle type was tested. While a maximum pressure of 600 bar or 9,000 psi may seem modest in current day UHPLC systems, a maximum operating temperature of 80–100 °C provides a degree of freedom that can be very beneficial, particularly when working with more hydrophobic proteins, including monoclonal antibodies, as will be illustrated in the following proof statements.
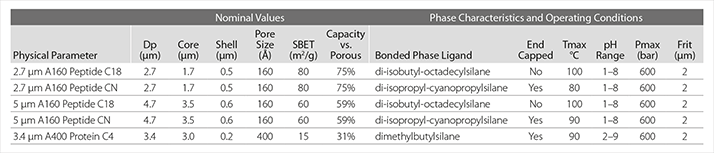 Table 1. Characteristics and Operating Conditions of BIOshell Fused-Core Columns
Table 1. Characteristics and Operating Conditions of BIOshell Fused-Core Columns




