Introduction
Antibody therapeutics are enjoying high growth rates in the biopharmaceutical market, the major areas of therapeutic application being cancer and immune/inflammation-related disorders including arthritis and multiple sclerosis. In 2010, four of the top ten best-selling global drug brands were monoclonal antibodies (mAbs). The characterization of these complex biomolecules is a major challenge in process monitoring and quality control. The main product characteristics to be monitored are aggregate and fragment content, glycosylation pattern and charged isoforms. The standard method used in biopharmaceutical QC for mAb aggregate and fragment analysis is size exclusion chromatography (SEC). A new series of silica based HPLC columns can be applied to either increase speed or improve resolution of the separation of antibody fragments, monomers and dimers.

Experimental
IgG was digested with papain over 24 hours. The fragmentation process was monitored by analyzing 10 or 5 μl aliquots of the sample. Mobile phase: 200 mmol/L phosphate buffer + 0.05% NaN3, pH 6.7
Flow rate: A & B: 1.0 ml/min C: 0.35 mL/min
Injection vol.: A & B: 10 μl; C: 5 μL
Temperature: 25°C
Detection: uV @280 nm
Samples: 10 g/L IgG digested with papain for 0-24 hr
Columns: A: TSKgel G3000SWXL, 7.8 mm ID x 30 cm
B: TSKgel SuperSW mAb Hr, 7.8 mm ID x 30 cm
C: TSKgel SuperSW mAb HTP, 4.6 mm ID x 15 cm
Results
Figure 1A shows the separation of a papain digested immunoglobulin G sample on a TSKgel G3000SWXL column, which is applied as the standard SEC column in routine analysis of aggregates in many QC and r&D labs. Figure 1B demonstrates that the resolution of the separation can be improved by using the new TSKgel SuperSW mAb Hr (Hr stands for ‘High resolution’) with 4 micron silica particles. This column provides higher resolution than the conventional column at the same analysis time. using the TSKgel SuperSW mAb HTP (HTP stands for ‘High Throughput’), a short semi-micro column packed with the same 4 micron particles as SuperSW mAb Hr, dimer/ monomer/ and fragments were separated at the same resolving power as on the conventional column but in half the analysis time (Figure 1C).
Separation of mAb Fragments, Monomers and Dimers
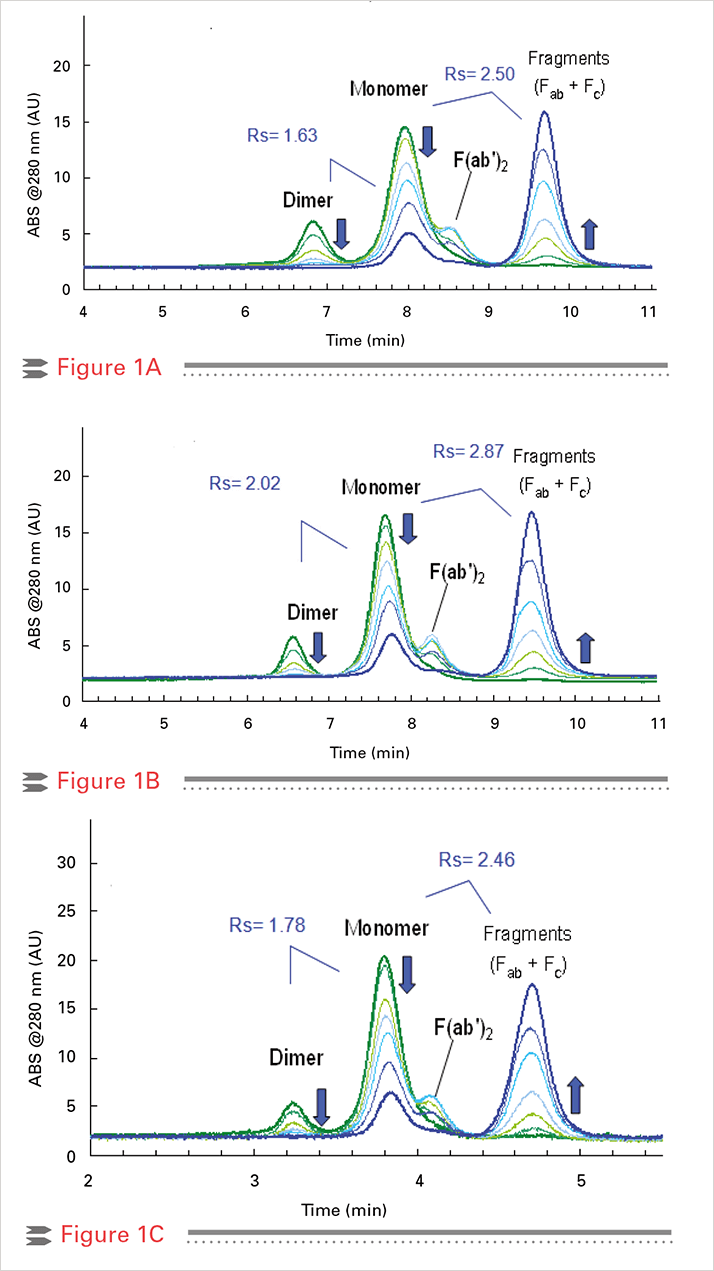 Separation of antibody fragments, monomers and dimers by SEC A: TSKgel G3000SWXL, 7.8 mm ID x 30 cm; B: TSKgel SuperSW mAb Hr, 7.8 mm ID x 30 cm C: TSKgel SuperSW mAb HTP, 4.6 mm ID x 15 cm
Separation of antibody fragments, monomers and dimers by SEC A: TSKgel G3000SWXL, 7.8 mm ID x 30 cm; B: TSKgel SuperSW mAb Hr, 7.8 mm ID x 30 cm C: TSKgel SuperSW mAb HTP, 4.6 mm ID x 15 cmSummary
Size exclusion chromatography (SEC) is a common method for the separation of antibody monomer from dimer, aggregates, or degradation products on the basis of molecular size. Two novel SEC columns designed for antibody separation exhibit reduced analysis time while achieving baseline separation or enhanced resolution between monomer and dimer.





