Introduction
Aeris WIDEPORE is a recently introduced core-shell HPLC | UHPLC column specifi cally designed to provide improved resolution of intact proteins larger than 10 kilodaltons (KDa) in molecular weight. The improved resolution of proteins is accomplished by the use of a new core-shell particle morphology which minimizes Protein band-spreading that occurs during diffusion in and out of the coreshell particle. The result is narrower peaks and better resolution of closely eluting proteins. This improved resolution is especially useful for refolding, impurity, and post-translational modifi cation assays on intact biogeneric proteins where very slight differences between intact and modifi ed proteins elute closely on reversed Phase columns. Several examples are shown demonstrating the utility of Aeris WIDEPORE core-shell columns for such applications.
 TN-1111 APPLICATIONS
TN-1111 APPLICATIONSSince their debut over two years ago, Kinetex® core-shell columns have introduced a new paradigm in ultra-high performance by decoupling column effi ciency from high backpressures. This has resulted in small molecule applications with reduced run times and increased throughput without the need for expensive new UHPLC instrumentation. While analysis speed has some value for protein separations, the principal focus is more on improving resolution of proteins from near-identical post-translationally modifi ed impurities rather than reducing run times. A new wide pore core-shell column specifi cally designed to improve protein separations (Aeris WIDEPORE) has been introduced. Rather than utilize a similar morphology of small molecule core-shell columns with larger pores, a completely different particle that takes into account the slower diffusion of proteins in and out of porous particles was developed. A graphic representation of the Aeris particle is shown in Figure 1.
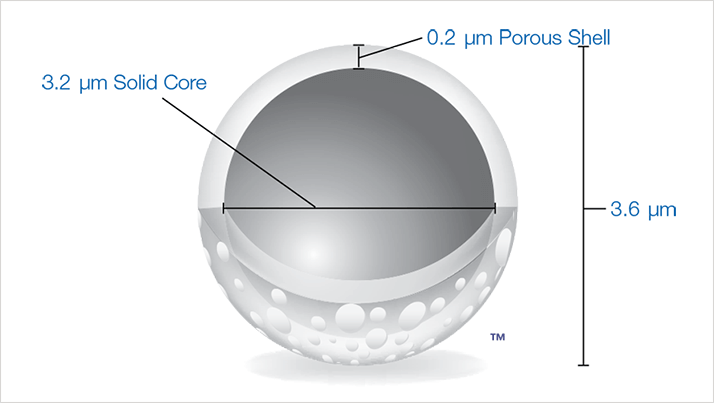 Figure 1. Graphical representation of Aeris 3.6 µm WIDEPORE particle. A 0.2 µm porous shell surrounds a 3.2 µm solid core. This particle geometry is specifi cally designed to narrow the peak width and improve resolution of proteins and other large molecules.
Figure 1. Graphical representation of Aeris 3.6 µm WIDEPORE particle. A 0.2 µm porous shell surrounds a 3.2 µm solid core. This particle geometry is specifi cally designed to narrow the peak width and improve resolution of proteins and other large molecules.By greatly reducing the path length of protein diffusion, Protein peaks tend to be narrower allowing for better resolution between intact proteins and their post-translationally modifi ed impurities. The larger particle size of Aeris WIDEPORE columns generates lower column backpressures than small particle fully porous wide pore columns. This lower column backpressure enables the use of longer columns for maximizing protein resolution.In recent years several therapeutic proteins have gone off patent, allowing a large number of organizations to develop their own generic versions of these potent therapeutics. With bioequivalence being an important aspect in the regulatory success of any biotherapeutic it is of the utmost importance for researchers to develop analytical methods that fully characterize and quantitate all of the impurities present in a candidate molecule. While peptide mapping is the more common method for identifying low-level post-translational modifi cations (PTMs), mapping gives only minimal Information about protein folding and can sometimes miss N-terminal and C-terminal modifi cations. Thus, intact protein analysis by reversed phase HPLC | UHPLC is usually part of the suite of testing performed on any protein therapeutic. The Aeris WIDEPORE coreshell column offers a new and improved solution for intact Protein analysis by offering narrower peak widths and improved Protein resolution when compared to fully porous wide pore media. This technical note will show several examples of using Aeris WIDEPORE for analyzing PTM’s on small to moderate proteins similar in size and chemical characteristics to several protein therapeutics. In addition, separations on common biogenerics will also be shown to demonstrate the improved performance of the Aeris WIDEPORE core-shell columns.
Materials and Methods
All chemical and standard proteins were obtained from Sigma Chemical (St. Louis, MO, USA). Recombinant human EGF and alpha interferon were purchased from R&D Systems (Minneapolis, MN, USA). Solvents were purchased from EMD (San Diego, CA, USA). Fully porous 300 Å C18 columns were purchased from various HPLC column vendors. Core-shell Aeris WIDEPORE 3.6 μm XB-C18 columns were obtained from Phenomenex (Torrance, CA, USA). Myoglobin samples were partially degraded by incubation at room temperature for up to a week in dilute acid. Ribonuclease samples were reduced with 100 mM DTT in 50 mM NH4HCO3 pH 8.0 for 20 minutes at 45 ºC; reduced/non-reduced mixtures were generated by spiking different ratios of the native to the reduced sample Prior to injection on HPLC. Different protein samples were analyzed on an Agilent® 1200 HPLC system with autosampler, column oven, solvent degasser, and UV detector set at 214 nm. Data was collected using ChemStation software (Agilent, Santa Clara, CA, USA). Mobile phases used were 0.1% TFA in water (A) and 0.085% TFA in acetonitrile (B). Different gradients, fl ow rates and column temperatures were listed with the corresponding chromatograms.





