Dextran is used as a blood plasma volume expander or blood flow improver. The dextran molar mass is crucial for the success of the therapy.
PSS WinGPC UniChrom is a macromolecular chromatography data system (MCDS) that offers comprehensive solutions for the characterization of macromolecules with GPC/SEC/GFC, interaction polymer chromatography (IPC) and 2-dimensional chromatography (2D) supporting all multi-detection techniques. Specific e-workflows in WinGPC guide scientists through special tasks such as United States Pharmacopeia (USP) and European Pharmacopoeia (EP) recommended low-molecular-weight (LMW) heparin calibration and analysis, sieve curve and MW cut-off determination or gelatin-specific calibration and multi-area analysis.

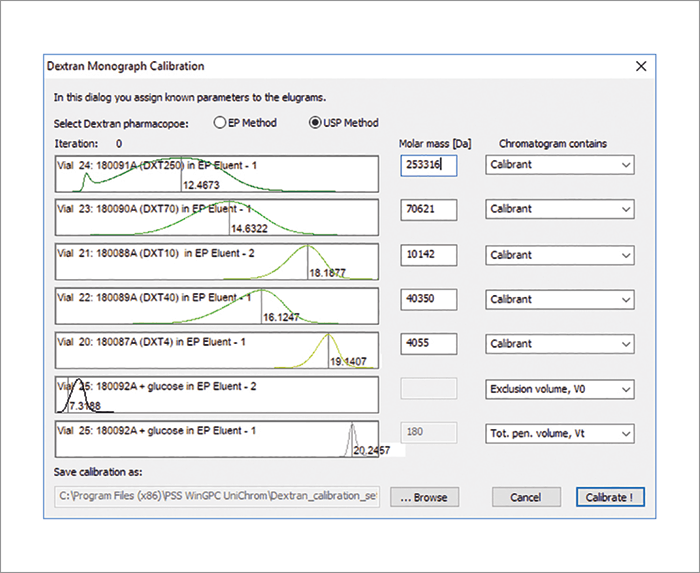
The latest addition to the e-workflows in WinGPC is the molecular weight determination of dextrans. A specific routine allows for calibration and molar mass determination for dextran 40, 60 and 70 as stated by USP and EP. USP/EP recommend aqueous size exclusion chromatography (SEC), with a specific dextran calibration to determine the molar mass information. Specific results for dextran samples include the weight average molar mass (Mw) for the whole dextran as well as for the fractions at 10 percent and 90 percent. USP additionally requires Mn and the polydispersity index (DI).
PSS WinGPC Software has a specific e-workflow implemented comprising data capture, specific dextran calibration, data analysis and compliant reporting. Regulated laboratories should opt for the WinGPC UniChrom Compliance Pack for FDA 21CFR11 support, including audit trails and electronic signatures.
The “Dextran Monograph Calibration” dialogue (Figure 1) uses five dextran standards of known molecular weights ranging from 4,000 to 250,000 Da, Glucose (180 Da, total column volume [Vt]) and a value for the V0 (column void volume) to start the iterative non-linear regression fit based on the concept by Nilsson and Nilsson. As the monographs differ slightly in their requirements, scientists have to choose between the EP method and the USP method. If the data can be successfully fitted, a WinGPC calibration file is saved and a report is printed.
Recorded data of the verification samples (System Suitability, Performance) and unknown dextrans can then be evaluated using the calibration curve created above and a specific menu item. WinGPC will automatically determine three different areas for the full dextran, 10 percent eluted mass and 90 percent eluted mass.





